Summary: A new study reports repeated stimulation enlarges dendritic spines, enabling contact with existing neural networks.
Source: Goethe University.
Even in adult brains, new neurons are generated throughout a lifetime. In a publication in the scientific journal PNAS, a research group led by Goethe University describes plastic changes of adult-born neurons in the hippocampus, a critical region for learning: frequent nerve signals enlarge the spines on neuronal dendrites, which in turn enables contact with the existing neural network.
Practise makes perfect, and constant repetition promotes the ability to remember. Researchers have been aware for some time that repeated electrical stimulation strengthens neuron connections (synapses) in the brain. It is similar to the way a frequently used trail gradually widens into a path. Conversely, if rarely used, synapses can also be removed – for example, when the vocabulary of a foreign language is forgotten after leaving school because it is no longer practised. Researchers designate the ability to change interconnections permanently and as needed as the plasticity of the brain.
Plasticity is especially important in the hippocampus, a primary region associated with long-term memory, in which new neurons are formed throughout life. The research groups led by Dr Stephan Schwarzacher (Goethe University), Professor Peter Jedlicka (Goethe University and Justus Liebig University in Gießen) and Dr Hermann Cuntz (FIAS, Frankfurt) therefore studied the long-term plasticity of synapses in new-born hippocampal granule cells. Synaptic interconnections between neurons are predominantly anchored on small thorny protrusions on the dendrites called spines. The dendrites of most neurons are covered with these spines, similar to the thorns on a rose stem.
In their recently published work, the scientists were able to demonstrate for the first time that synaptic plasticity in new-born neurons is connected to long-term structural changes in the dendritic spines: repeated electrical stimulation strengthens the synapses by enlarging their spines. A particularly surprising observation was that the overall size and number of spines did not change: when the stimulation strengthened a group of synapses, and their dendritic spines enlarged, a different group of synapses that were not being stimulated simultaneously became weaker and their dendritic spines shrank.
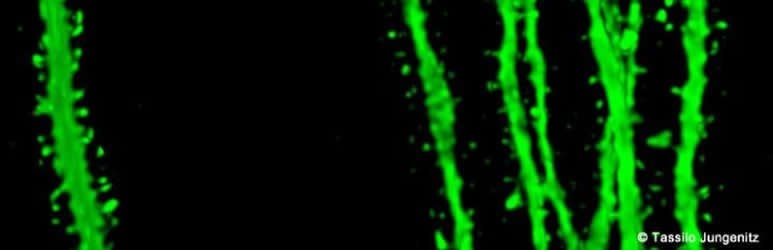
“This observation was only technically possible because our students Tassilo Jungenitz and Marcel Beining succeeded for the first time in examining plastic changes in stimulated and non-stimulated dendritic spines within individual new-born cells using 2-photon microscopy and viral labelling,” says Stephan Schwarzacher from the Institute for Anatomy at the University Hospital Frankfurt. Peter Jedlicka adds: “The enlargement of stimulated synapses and the shrinking of non-stimulated synapses was at equilibrium. Our computer models predict that this is important for maintaining neuron activity and ensuring their survival.”
The scientists now want to study the impenetrable, spiny forest of new-born neuron dendrites in detail. They hope to better understand how the equilibrated changes in dendritic spines and their synapses contribute the efficient storing of information and consequently to learning processes in the hippocampus.
Funding: The work described in this article was partially funded by the EU H2020 TOXI-Triage Project #653409.
Source: Stephan Schwarzacher – Goethe University
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Tassilo Jungenitz.
Original Research: Abstract for “Structural homo- and heterosynaptic plasticity in mature and adult newborn rat hippocampal granule cells” by Tassilo Jungenitz, Marcel Beining, Tijana Radic, Thomas Deller, Hermann Cuntz, Peter Jedlicka, and Stephan W. Schwarzacher in PNAS. Published May 15 2018
doi:10.1073/pnas.1801889115
[cbtabs][cbtab title=”MLA”]Goethe University “Thorny Life of New Born Neurons.” NeuroscienceNews. NeuroscienceNews, 10 June 2018.
<https://neurosciencenews.com/dendritic-spine-enlargement-9304/>.[/cbtab][cbtab title=”APA”]Goethe University (2018, June 10). Thorny Life of New Born Neurons. NeuroscienceNews. Retrieved June 10, 2018 from https://neurosciencenews.com/dendritic-spine-enlargement-9304/[/cbtab][cbtab title=”Chicago”]Goethe University “Thorny Life of New Born Neurons.” https://neurosciencenews.com/dendritic-spine-enlargement-9304/ (accessed June 10, 2018).[/cbtab][/cbtabs]
Abstract
Structural homo- and heterosynaptic plasticity in mature and adult newborn rat hippocampal granule cells
Adult newborn hippocampal granule cells (abGCs) contribute to spatial learning and memory. abGCs are thought to play a specific role in pattern separation, distinct from developmentally born mature GCs (mGCs). Here we examine at which exact cell age abGCs are synaptically integrated into the adult network and which forms of synaptic plasticity are expressed in abGCs and mGCs. We used virus-mediated labeling of abGCs and mGCs to analyze changes in spine morphology as an indicator of plasticity in rats in vivo. High-frequency stimulation of the medial perforant path induced long-term potentiation in the middle molecular layer (MML) and long-term depression in the nonstimulated outer molecular layer (OML). This stimulation protocol elicited NMDA receptor-dependent homosynaptic spine enlargement in the MML and heterosynaptic spine shrinkage in the inner molecular layer and OML. Both processes were concurrently present on individual dendritic trees of abGCs and mGCs. Spine shrinkage counteracted spine enlargement and thus could play a homeostatic role, normalizing synaptic weights. Structural homosynaptic spine plasticity had a clear onset, appearing in abGCs by 28 d postinjection (dpi), followed by heterosynaptic spine plasticity at 35 dpi, and at 77 dpi was equally as present in mature abGCs as in mGCs. From 35 dpi on, about 60% of abGCs and mGCs showed significant homo- and heterosynaptic plasticity on the single-cell level. This demonstration of structural homo- and heterosynaptic plasticity in abGCs and mGCs defines the time course of the appearance of synaptic plasticity and integration for abGCs.