Summary: Blood vessels can sense the metabolic state of nearby neurons. An imbalance of fatty acids is sensed by neural blood vessels, stimulating them to mount a stress response by loosening the blood-brain barrier. If the imbalance remains, the leaky blood-brain barrier can induce a disease state.
Source: Max Planck Institute
The brain is our most energy-hungry and metabolically active organ. It is responsible for our thoughts, ideas, movement and ability to learn. Our brain is powered by 600 km of blood vessels that bring it nutrients and remove waste products. However, the brain is also very fragile. Thus, the blood vessels in the brain have evolved to form a tight protective barrier – the blood-brain barrier – that restrict the movement of molecules in and out of the brain. It is essential that the brain can regulate its environment. On the one hand, pathogens or toxins are effectively prevented from entering the brain, but on the other hand, required messengers or nutrients can pass through them unhindered.
Epigenetics turns on the nutrition program
Given their close relationship, it is important that the brain and its vessels talk extensively to one another. Recent work in the lab of Asifa Akhtar in Freiburg has shown that blood vessels can sense the metabolic state of neighboring neural cells.
The researchers found that the epigenetic regulator MOF is required for equipping neurons with the right metabolic enzymes needed for processing fatty acids. “Something has to tell neural cells that there are nutrients around and they should turn on the programs needed to process them,” explains Bilal Sheikh, lead author of the study. “MOF goes to the DNA and switches on the genetic programs that allow cells to process fatty acids in the brain”.
Fatty acids are found in food and are used for generating energy and assembling complex lipids required in cell membranes. When the activity of MOF is defective, as occurs in neural developmental disorders, the neurons cannot process fatty acids. This leads to their accumulation in the interstitial spaces between the brain cells. In their studies, Asifa Akhtar’s team uncovered that this imbalance in fatty acids is sensed by the neural blood vessels, stimulating them to mount a stress response by loosening the blood-brain barrier. If the metabolic imbalance remains, the leaky blood-brain barrier can induce a diseased state.
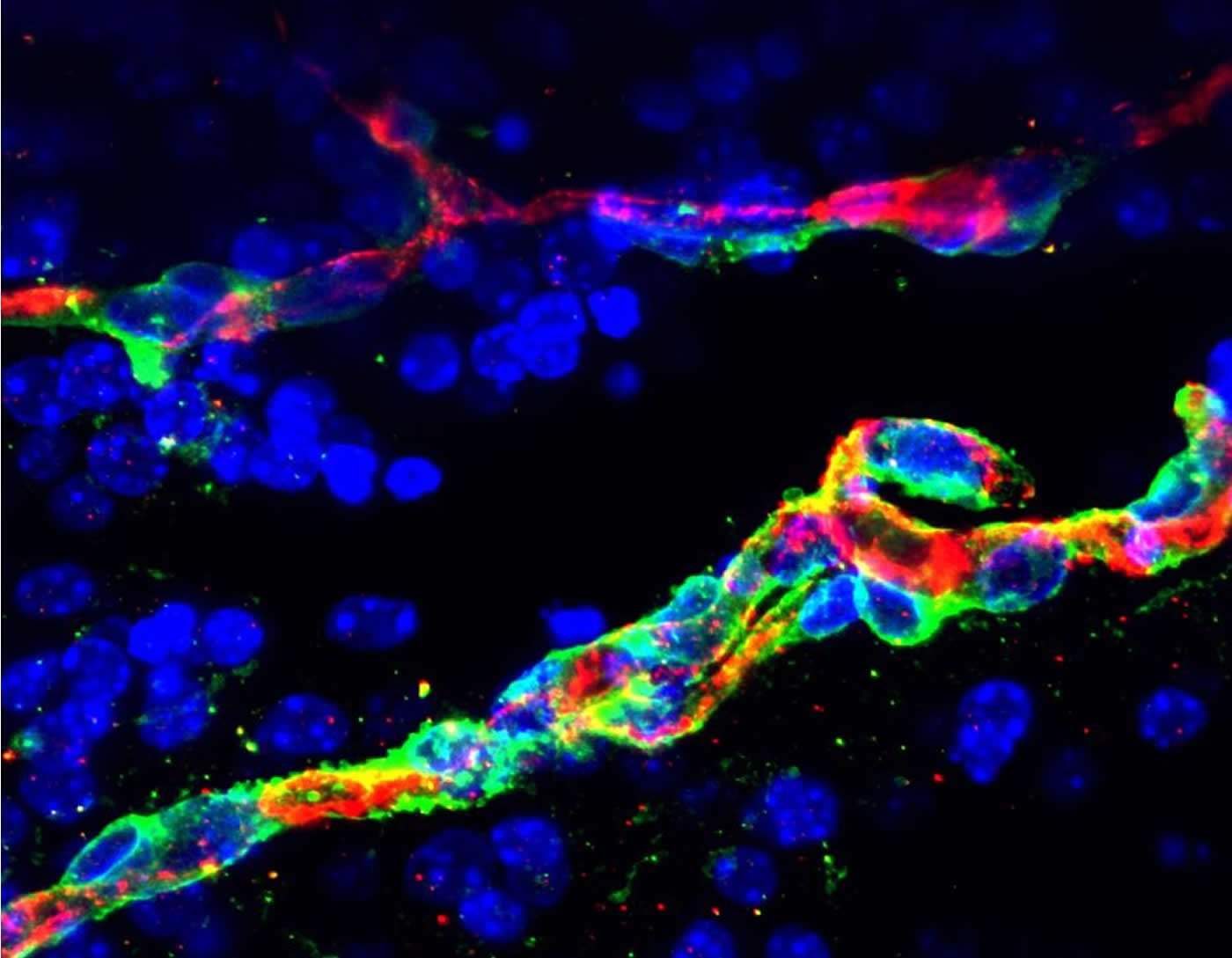
Neural blood vessel breakdown
The study sets the foundation for a better understanding of how neural cells and blood vessels talk to each other in the brain and illustrates how changes in the metabolic milieu of one cell type in a complex organ can directly impact the functionality of surrounding cells and thereby affect overall organ function. “Our work shows that proper metabolism in the brain is critical for its health. A defective neural metabolic environment can induce vascular inflammation, dysfunction of the cells forming the blood-brain barrier, and increased permeability. What can follow is neural blood vessel breakdown,” explains Asifa Akhtar. This is particularly important, as neural blood vessel breakdown is a characteristic feature of the onset of age-related diseases such as Alzheimer’s disease and vascular dementia. Better characterization of the molecular changes that induce vascular dysfunction will help design better treatments for these debilitating pathologies.
About this neuroscience research article
Source:
Max Planck Institute
Media Contacts:
Marcus Rockoff – Max Planck Institute
Image Source:
The image is credited to MPI of Immunobiology and Epigenetics, B. Sheikh.
Original Research: Closed access
“Neural metabolic imbalance induced by MOF dysfunction triggers pericyte activation and breakdown of vasculature”. by Bilal N. Sheikh, Sukanya Guhathakurta, Tsz Hong Tsang, Marius Schwabenland, Gina Renschler, Benjamin Herquel, Vivek Bhardwaj, Herbert Holz, Thomas Stehle, Olga Bondareva, Nadim Aizarani, Omar Mossad, Oliver Kretz, Wilfried Reichardt, Aindrila Chatterjee, Laura J. Braun, Julien Thevenon, Herve Sartelet, Thomas Blank, Dominic Grün, Dominik von Elverfeldt, Tobias B. Huber, Dietmar Vestweber, Sergiy Avilov, Marco Prinz, Joerg M. Buescher & Asifa Akhtar.
Nature Cell Biology doi:10.1038/s41556-020-0526-8
Abstract
Neural metabolic imbalance induced by MOF dysfunction triggers pericyte activation and breakdown of vasculature
Mutations in chromatin-modifying complexes and metabolic enzymes commonly underlie complex human developmental syndromes affecting multiple organs. A major challenge is to determine how disease-causing genetic lesions cause deregulation of homeostasis in unique cell types. Here we show that neural-specific depletion of three members of the non-specific lethal (NSL) chromatin complex—Mof, Kansl2 or Kansl3—unexpectedly leads to severe vascular defects and brain haemorrhaging. Deregulation of the epigenetic landscape induced by the loss of the NSL complex in neural cells causes widespread metabolic defects, including an accumulation of free long-chain fatty acids (LCFAs). Free LCFAs induce a Toll-like receptor 4 (TLR4)–NFκB-dependent pro-inflammatory signalling cascade in neighbouring vascular pericytes that is rescued by TLR4 inhibition. Pericytes display functional changes in response to LCFA-induced activation that result in vascular breakdown. Our work establishes that neurovascular function is determined by the neural metabolic environment.