Summary: Alzheimer’s linked protein, amyloid beta, appears to do very little harm to glial cells, at least in fruit flies, researchers report.
Source: Linköping University.
Amyloid beta, a protein linked with Alzheimer’s disease, has different properties in different cell types in the brains of fruit flies. This is the conclusion of a study led by researchers at Linköping University in Sweden. While amyloid beta is highly toxic for nerve cells, it seems that certain other types of cell are hardly damaged at all by aggregates of the protein.
The study, which has been published in Cell Chemical Biology, describes investigations by Swedish researchers into the sensitivity of different cells in the brain for one of the proteins closely associated with Alzheimer’s disease. In advanced Alzheimer’s disease, huge numbers of nerve cells in the brain are dead. Research has long been focused onto the process by which nerve cells are damaged by erroneously folded forms of the amyloid beta protein. These forms build up and eventually form plaques in the brain. But the erroneously folded forms of amyloid beta do not accumulate only in nerve cells. Amyloid deposits are also found in the blood vessels of the brain, in the retina, and in cells known as glial cells. The latter have various support functions in the brain, and it is unclear whether this plays a role in the development of disease. For this reason, the researchers wanted to investigate whether amyloid beta can form in these different types of cell, and whether it is toxic for other cells than nerve cells.
The researchers used fruit flies (Drosophila melanogaster) in their work. These have been extensively used in research to understand neuronal development and diseases, including Alzheimer’s disease. They used fruit flies that had been modified, such that their cells produced high levels of human amyloid beta 1-42, which is the more harmful of the two most common variants. The researchers could control which cells expressed the amyloid, and compared flies in which it was expressed in different cell types. The group had previously shown that the higher the amount of amyloid aggregate present in the nerve cells, the more severe was the disease in the flies.
“In this study we expressed the amyloid beta 1-42 in glial cells instead, and observed that huge amounts of aggregate accumulated around these cells. The flies, however, were hardly affected by the disease. They were affected to a certain degree, compared with control groups, but nowhere like as much as flies with amyloid beta in their nerve cells. This was a great surprise,” says Maria Jonson, research student in the Department of Physics, Chemistry and Biology and first author of the article.
The researchers wondered why amyloid did not harm the glial cells as much as nerve cells, and thus studied the structure of the aggregate in detail. Amyloid beta with faulty folding can be produced in various forms, and these are classified by, among other things, the degree of maturity. Mature amyloid appears in the microscope as thin, tightly packed strands, almost like a bundle of uncooked spaghetti. When immature, it looks more like cooked spaghetti, and forms tangles. Previous studies by the researchers in mice and humans have shown that both forms can be present, but this is the first time that neuron degradation was linked to the structure of the amyloid.
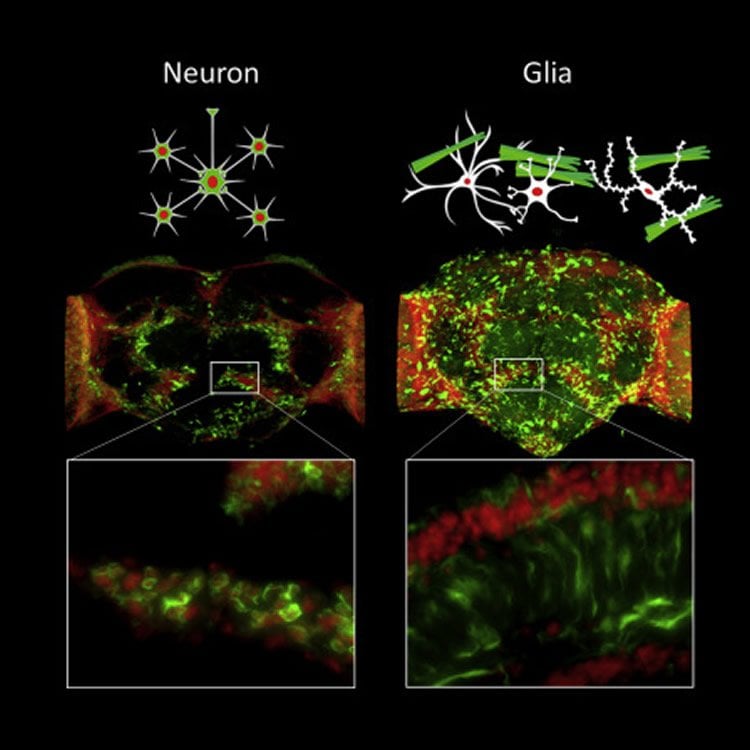
“We noted that glial cells seem to produce the mature, less harmful form of amyloid beta, while neurons cannot. The amyloid ends up outside the glial cells as bundles of fibres, while the same protein in its immature form gets stuck inside the neurons, and they die. This raises the question, of course, of the molecular mechanism that lies behind the high toxicity of amyloid beta for neurons, while glial cells can survive even with high levels, at least in fruit flies,” says Per Hammarström, professor in the Department of Physics, Chemistry and Biology, and leader of the study.
One important advantage of using fruit flies as experimental model, rather than mice, is that high levels of the amyloid beta aggregate in the flies leads to neurodegeneration and a considerably shorter lifetime, which is the same as in humans. Stefan Thor’s research group at the Department of Clinical and Experimental Medicine has developed the fruit flies used in the study.
Funding: The research has been financed with support of, among other sources, The Swedish Brain Foundation (Hjärnfonden), the Swedish Research Council, the Swedish Alzheimer’s Foundation, and Göran Gustafsson’s Foundation.
Source: Leah Russell – Linköping University
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Jonson et al./Cell Chemical Biology.
Original Research: Abstract for “Aggregated Aβ1-42 is selectively toxic for neurons, whereas glial cells produce mature fibrils with low toxicity in Drosophila” by Maria Jonson, Sofie Nyström, Alexander Sandberg, Marcus Carlback, Wojciech Michno, Jörg Hanrieder, Annika Starkenberg, K. Peter R. Nilsson, Stefan Thor and Per Hammarström in Cell Chemical Biology. Published April 12 2018.
doi:10.1016/j.chembiol.2018.03.006
[cbtabs][cbtab title=”MLA”]Linköping University “Alzheimer’s Plaque Affects Different Brain Cells Differently.” NeuroscienceNews. NeuroscienceNews, 14 April 2018.
<https://neurosciencenews.com/amyloid-beta-neurons-8788/>.[/cbtab][cbtab title=”APA”]Linköping University (2018, April 14). Alzheimer’s Plaque Affects Different Brain Cells Differently. NeuroscienceNews. Retrieved April 14, 2018 from https://neurosciencenews.com/amyloid-beta-neurons-8788/[/cbtab][cbtab title=”Chicago”]Linköping University “Alzheimer’s Plaque Affects Different Brain Cells Differently.” https://neurosciencenews.com/amyloid-beta-neurons-8788/ (accessed April 14, 2018).[/cbtab][/cbtabs]
Abstract
Aggregated Aβ1-42 is selectively toxic for neurons, whereas glial cells produce mature fibrils with low toxicity in Drosophila
Highlights
•Expressed Aβ1-42 aggregates profoundly in various cell types of Drosophila
•Aβ1-42 accumulates as extracellular amyloid fibrils when expressed in glial cells
•Aβ1-42 is highly toxic to neurons in Drosophila
•Immature intracellular aggregates are more toxic than mature fibrillar Aβ1-42
Summary
The basis for selective vulnerability of certain cell types for misfolded proteins (MPs) in neurodegenerative diseases is largely unknown. This knowledge is crucial for understanding disease progression in relation to MPs spreading in the CNS. We assessed this issue in Drosophila by cell-specific expression of human Aβ1-42 associated with Alzheimer’s disease. Expression of Aβ1-42 in various neurons resulted in concentration-dependent severe neurodegenerative phenotypes, and intraneuronal ring-tangle-like aggregates with immature fibril properties when analyzed by aggregate-specific ligands. Unexpectedly, expression of Aβ1-42 from a pan-glial driver produced a mild phenotype despite massive brain load of Aβ1-42 aggregates, even higher than in the strongest neuronal driver. Glial cells formed more mature fibrous aggregates, morphologically distinct from aggregates found in neurons, and was mainly extracellular. Our findings implicate that Aβ1-42 cytotoxicity is both cell and aggregate morphotype dependent.






