Summary: Study finds a ‘peculiar association’ between amyloid precursor protein and cholesterol in the cell membrane of synapses. Amyloid precursor protein may contribute to cholesterol deficiency, triggering neurodegeneration in Alzheimer’s disease.
Source: Florida Atlantic University
Worldwide, 50 million people are living with Alzheimer’s disease and other dementias. According to the Alzheimer’s Association, every 65 seconds someone in the United States develops this disease, which causes problems with memory, thinking and behavior.
It has been more than 100 years since Alois Alzheimer, M.D., a German psychiatrist and neuropathologist, first reported the presence of senile plaques in an Alzheimer’s disease patient brain. It led to the discovery of amyloid precursor protein that produces deposits or plaques of amyloid fragments in the brain, the suspected culprit of Alzheimer’s disease. Since then, amyloid precursor protein has been extensively studied because of its association with Alzheimer’s disease. However, amyloid precursor protein distribution within and on neurons and its function in these cells remain unclear.
A team of neuroscientists led by Florida Atlantic University’s Brain Institute sought to answer a fundamental question in their quest to combat Alzheimer’s disease — “Is amyloid precursor protein the mastermind behind Alzheimer’s disease or is it just an accomplice?”
Mutations found in amyloid precursor protein have been linked to rare cases of familial Alzheimer’s disease. Although scientists have gained a lot knowledge about how this protein turns into amyloid plaques, little is known about its native function in neurons. In the case of more common sporadic Alzheimer’s disease, the highest genetic risk factor is a protein that is involved in cholesterol transportation and not this amyloid precursor protein. Moreover, various clinical trials designed to address Alzheimer’s disease by minimizing amyloid plaque formation have failed, including one from Biogen announced last month.
In a study published in the journal Neurobiology of Disease, Qi Zhang, Ph.D., senior author, an investigator at the FAU Brain Institute, and an assistant research professor in FAU’s Schmidt College of Medicine, along with collaborators from Vanderbilt University, tackle this Alzheimer’s disease mystery by devising a multi-functional reporter for amyloid precursor protein and tracking the protein’s localization and mobility using quantitative imaging with unprecedented accuracy.
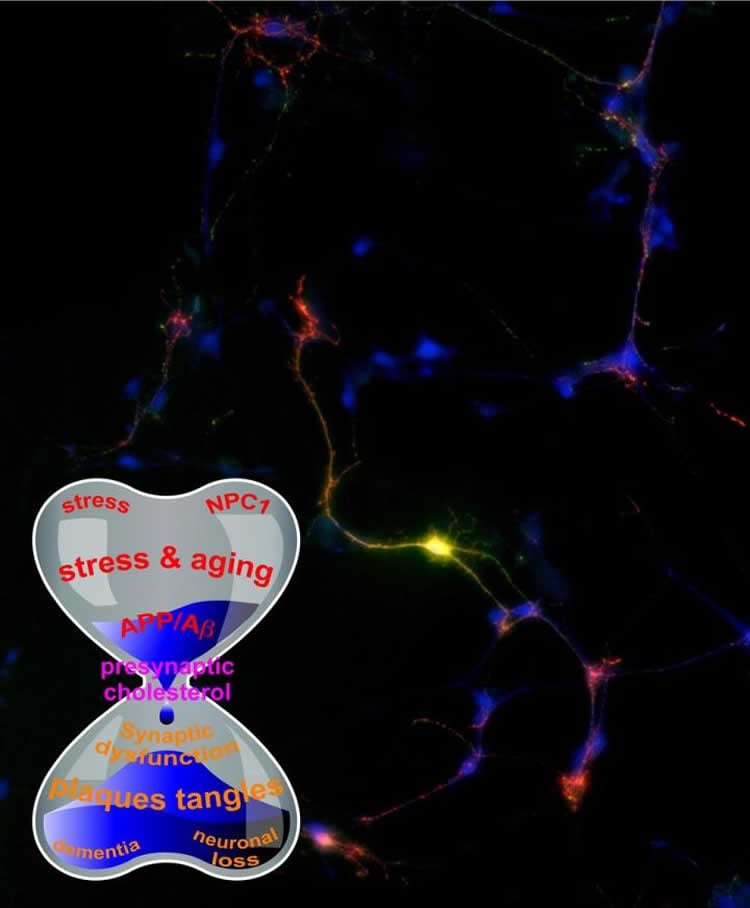
For the study, Zhang and collaborators genetically disrupted the interaction between cholesterol and amyloid precursor protein. Surprisingly, by disengaging the two, they discovered that this manipulation not only disrupts the trafficking of amyloid precursor protein but also messes up cholesterol distribution at the neuronal surface. Neurons with an altered distribution of cholesterol exhibited swollen synapses and fragmented axons and other early signs of neurodegeneration.
“Our study is intriguing because we noticed a peculiar association between amyloid precursor protein and cholesterol that resides in the cell membrane of synapses, which are points of contact among neurons and the biological basis for learning and memory,” said Zhang.
“Amyloid precursor protein may just be one of the many accomplices partially contributing to cholesterol deficiency. Strangely, the heart and brain seem to meet again in the fight against bad cholesterol.”
Given the broad involvement of cholesterol in almost all aspects of neurons’ life, Zhang and collaborators have proposed a new theory about the amyloid precursor protein connection in Alzheimer’s disease, especially in the surface of those tiny synapses, which triggers neurodegeneration.
“Although still in early stages, this cutting-edge research by Dr. Zhang and his collaborators at Vanderbilt University may have implications for the millions of people at risk for or suffering with Alzheimer’s disease,” said Randy D. Blakely, Ph.D., executive director of the FAU Brain Institute and a professor of biomedical science in FAU’s Schmidt College of Medicine. “The number of people in Florida alone who are age 65 and older with Alzheimer’s disease is expected to increase 41.2 percent by 2025 to a projected 720,000, highlighting the urgency of finding a medical breakthrough.”
Locally, Alzheimer’s disease affects 11.5 percent of Medicare beneficiaries in Palm Beach County and 12.7 percent of Medicare beneficiaries in Broward County (a nearly 18 percent increase over national average).
According to the Alzheimer’s Association, Florida is number one in per capita cases of Alzheimer’s disease in the U.S.
Study co-authors of “Reciprocal Modulation between Amyloid Precursor Protein and Synaptic Membrane Cholesterol Revealed by Live Cell Imaging,” are Claire E. DelBove and Claire E. Strothman, graduate students; Roman M. Lazarenko, Ph.D., a post-doctoral fellow; Hui Huang, Ph.D.; and Charles R. Sanders, Ph.D., associate dean for research and a professor of biochemistry, all with Vanderbilt University.
Funding: This research is funded by the National Institutes of Health (OD00876101 and NS094738 awarded to Zhang and R01 AG056147 awarded to Sanders) and the FAU Brain Institute.
Source:
Florida Atlantic University
Media Contacts:
Gisele Galoustian – Florida Atlantic University
Image Source:
The image is credited to Qi Zhang, Ph.D. and Claire E. DelBove.
Original Research: Closed access
“Reciprocal modulation between amyloid precursor protein and synaptic membrane cholesterol revealed by live cell imaging”
Claire E. DelBove, Claire E. Strothman, Roman M. Lazarenko, Hui Huang, Charles R. Sanders, Qi Zhang. Neurobiology of Disease doi:10.1016/j.nbd.2019.03.009
Abstract
Reciprocal modulation between amyloid precursor protein and synaptic membrane cholesterol revealed by live cell imaging
The amyloid precursor protein (APP) has been extensively studied because of its association with Alzheimer’s disease (AD). However, APP distribution across different subcellular membrane compartments and its function in neurons remains unclear. We generated an APP fusion protein with a pH-sensitive green fluorescent protein at its ectodomain and a pH-insensitive blue fluorescent protein at its cytosolic domain and used it to measure APP’s distribution, subcellular trafficking, and cleavage in live neurons. This reporter, closely resembling endogenous APP, revealed only a limited correlation between synaptic activities and APP trafficking. However, the synaptic surface fraction of APP was increased by a reduction in membrane cholesterol levels, a phenomenon that involves APP’s cholesterol-binding motif. Mutations at or near binding sites not only reduced both the surface fraction of APP and membrane cholesterol levels in a dominant negative manner, but also increased synaptic vulnerability to moderate membrane cholesterol reduction. Our results reveal reciprocal modulation of APP and membrane cholesterol levels at synaptic boutons.






