We have known for some years that Alzheimer’s disease is characterised by two types of lesions, amyloid plaques and degenerated tau protein. Cholesterol plays an important role in the physiopathology of this disease. Two French research teams (Inserm/CEA/University of Lille/University of Paris-Sud ) have just shown, in a rodent model, that overexpressing an enzyme that can eliminate excess cholesterol from the brain may have a beneficial action on the tau component of the disease, and completely correct it. This is the first time that a direct relationship has been shown between the tau component of Alzheimer’s disease and cholesterol. This work is published in the 10 September 2015 issue of Human Molecular Genetics.
xcess brain cholesterol cannot freely cross the blood-brain barrier; to be eliminated it must be converted into 24-hydroxycholesterol (24-OHC) by the enzyme CYP46A1 (cholesterol-24-hydroxylase). At Inserm Unit 1169, Nathalie, Cartier, coordinator of this work, and Patrick Aubourg, director of the unit, proposed the hypothesis that increasing the efflux of cholesterol from the brain by overexpressing CYP46A1 might have a beneficial effect on the elements of Alzheimer pathology.
The first step in this work made it possible to show that injecting a viral vector, AAV-CYP46A1, effectively corrects a mouse model of amyloid pathology of the disease, the APP23 mouse. CYP46A1 thus appears to be a therapeutic target for Alzheimer’s disease.
Conversely, in vivo inhibition of CYP46A1 in the mice, using antisense RNA molecules delivered by an AAV vector administered to the hippocampus, induces an increase in the production of Aß peptides, abnormal tau protein, neuronal death and hippocampal atrophy, leading to memory problems. Together these elements reproduce a phenotype mimicking Alzheimer’s disease.
These results demonstrate the key role of cholesterol in the disease, and confirm the relevance of CYP46A1 as a potential therapeutic target (work published in Brain on 3 July 2015).
Taken together, this work now enables the research team coordinated by Nathalie Cartier, Inserm Research Director, to propose a gene therapy approach for Alzheimer’s disease: intracerebral administration of a vector, AAV-CYP46A1, in patients with early and severe forms (1% of patients, familial forms) for whom there is no available treatment.
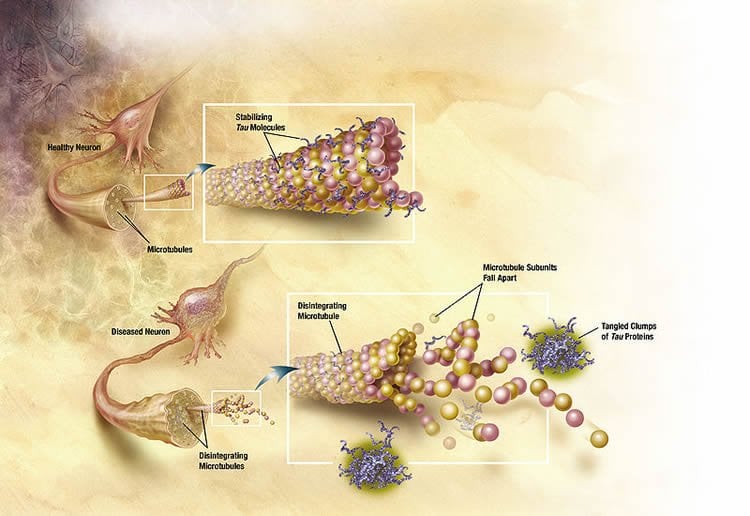
“To achieve this objective, we are carrying out all the preclinical steps of development and validation of the tools (vector, neurosurgical protocol, elements of monitoring) for demonstrating the efficacy and tolerance of the strategy, in order to submit an application for authorisation of a clinical trial,” explains Nathalie Cartier.
Other investigators on the study include Lauren Shields, Bryce Mendelsohn, Dominik Haddad, Wei Lin, and Hwajin Kim from the Gladstone Institutes; Akos Gerencser and Martin Brand from the Buck Institute for Research on Aging; and Robert Edwards from the University of California San Francisco.
Funding: The study was supported by grants from the National Institutes of Health, the Burroughs Wellcome Fund, the National Science Foundation, the Pediatric Scientist Development Program and from the Betty Brown Family, and the Joan and David Traitel Family Trust.
Source: Nathalie Cartier – INSERM
Image Credit: Image is credited to NIA/NIH and is in the public domain
Original Research: Abstract for “CYP46A1 inhibition, brain cholesterol accumulation and neurodegeneration pave the way for Alzheimer’s disease” by Fathia Djelti, Jerome Braudeau, Eloise Hudry, Marc Dhenain, Jennifer Varin, Ivan Bièche, Catherine Marquer, Farah Chali, Sophie Ayciriex, Nicolas Auzeil, Sandro Alves, Dominique Langui, Marie-Claude Potier, Olivier Laprevote, Michel Vidaud, Charles Duyckaerts, Richard Miles, Patrick Aubourg, and Nathalie Cartier in Brain. Published online July 3 2015 doi:10.1093/brain/awv166
Abstract for “Cholesterol 24-hydroxylase defect is implicated in memory impairments associated with Alzheimer-like tau pathology” by Marie-Anne Burlot, Jerome Braudeau, Kristin Michaelsen-Preusse, Brigitte Potier, Sophie Ayciriex, Jennifer Varin, Benoit Gautier, Fathia Djelti, Mickael Audrain, Luce Dauphinot, Francisco-Jose Fernandez-Gomez, Raphaelle Caillierez, Olivier Laprevote, Ivan Bi.che, Nicolas Auzeil, Marie-Claude Potier, Patrick Dutar, Martin Korte, Luc Buée, David Blum and Nathalie Cartier in Human Molecular Genetics. Published online September 10 2015 doi:10.1093/hmg/ddv268
Abstract
CYP46A1 inhibition, brain cholesterol accumulation and neurodegeneration pave the way for Alzheimer’s disease
Abnormalities in neuronal cholesterol homeostasis have been suspected or observed in several neurodegenerative disorders including Alzheimer’s disease, Parkinson’s disease and Huntington’s disease. However, it has not been demonstrated whether an increased abundance of cholesterol in neurons in vivo contributes to neurodegeneration. To address this issue, we used RNA interference methodology to inhibit the expression of cholesterol 24-hydroxylase, encoded by the Cyp46a1 gene, in the hippocampus of normal mice. Cholesterol 24-hydroxylase controls cholesterol efflux from the brain and thereby plays a major role in regulating brain cholesterol homeostasis. We used an adeno-associated virus vector encoding short hairpin RNA directed against the mouse Cyp46a1 mRNA to decrease the expression of the Cyp46a1 gene in hippocampal neurons of normal mice. This increased the cholesterol concentration in neurons, followed by cognitive deficits and hippocampal atrophy due to apoptotic neuronal death. Prior to neuronal death, the recruitment of the amyloid protein precursor to lipid rafts was enhanced leading to the production of β-C-terminal fragment and amyloid-β peptides. Abnormal phosphorylation of tau and endoplasmic reticulum stress were also observed. In the APP23 mouse model of Alzheimer’s disease, the abundance of amyloid-β peptides increased following inhibition of Cyp46a1 expression, and neuronal death was more widespread than in normal mice. Altogether, these results suggest that increased amounts of neuronal cholesterol within the brain may contribute to inducing and/or aggravating Alzheimer’s disease.
“CYP46A1 inhibition, brain cholesterol accumulation and neurodegeneration pave the way for Alzheimer’s disease” by Fathia Djelti, Jerome Braudeau, Eloise Hudry, Marc Dhenain, Jennifer Varin, Ivan Bièche, Catherine Marquer, Farah Chali, Sophie Ayciriex, Nicolas Auzeil, Sandro Alves, Dominique Langui, Marie-Claude Potier, Olivier Laprevote, Michel Vidaud, Charles Duyckaerts, Richard Miles, Patrick Aubourg, and Nathalie Cartier in Brain. Published online July 3 2015 doi:10.1093/brain/awv166
Abstract
Cholesterol 24-hydroxylase defect is implicated in memory impairments associated with Alzheimer-like tau pathology
Alzheimer’s disease (AD) is characterized by both amyloid and Tau pathologies. The amyloid component and altered cholesterol metabolism are closely linked, but the relationship between Tau pathology and cholesterol is currently unclear. Brain cholesterol is synthesized in situ and cannot cross the blood–brain barrier: to be exported from the central nervous system into the blood circuit, excess cholesterol must be converted to 24S-hydroxycholesterol by the cholesterol 24-hydroxylase encoded by the CYP46A1 gene. In AD patients, the concentration of 24S-hydroxycholesterol in the plasma and the cerebrospinal fluid are lower than in healthy controls. The THY-Tau22 mouse is a model of AD-like Tau pathology without amyloid pathology. We used this model to investigate the potential association between Tau pathology and CYP46A1 modulation. The amounts of CYP46A1 and 24S-hydroxycholesterol in the hippocampus were lower in THY-Tau22 than control mice. We used an adeno-associated virus (AAV) gene transfer strategy to increase CYP46A1 expression in order to investigate the consequences on THY-Tau22 mouse phenotype. Injection of the AAV-CYP46A1 vector into the hippocampus of THY-Tau22 mice led to CYP46A1 and 24S-hydroxycholesterol content normalization. The cognitive deficits, impaired long-term depression and spine defects that characterize the THY-Tau22 model were completely rescued, whereas Tau hyperphosphorylation and associated gliosis were unaffected. These results argue for a causal link between CYP46A1 protein content and memory impairments that result from Tau pathology. Therefore, CYP46A1 may be a relevant therapeutic target for Tauopathies and especially for AD.
“Cholesterol 24-hydroxylase defect is implicated in memory impairments associated with Alzheimer-like tau pathology” by Marie-Anne Burlot, Jerome Braudeau, Kristin Michaelsen-Preusse, Brigitte Potier, Sophie Ayciriex, Jennifer Varin, Benoit Gautier, Fathia Djelti, Mickael Audrain, Luce Dauphinot, Francisco-Jose Fernandez-Gomez, Raphaelle Caillierez, Olivier Laprevote, Ivan Bi.che, Nicolas Auzeil, Marie-Claude Potier, Patrick Dutar, Martin Korte, Luc Buée, David Blum and Nathalie Cartier in Human Molecular Genetics. Published online September 10 2015 doi:10.1093/hmg/ddv268