In biology, stability is important. From body temperature to blood pressure and sugar levels, our body ensures that these remain within reasonable limits and do not reach potentially damaging extremes. Neurons in the brain are no different and, in fact, have developed a number of ways to stabilise their electrical activity so as to avoid becoming either overexcitable, potentially leading to epilepsy, or not excitable enough, leading to non functional neurons. A new study published in PNAS by researchers from the MRC Centre for Developmental Neurobiology characterises a novel way in which neurons remain electrically stable when confronted with chronic increases in neuronal activity.
The site at the centre of this control mechanism is a short segment of the axon, where electrical activity is initiated in the first place. Also known as the axon initial segment (or AIS for short), this remarkable structure is responsible for integrating all the information that a neuron receives via its synapses to produce an action potential, the electrical currency of information used by neurons. It is perhaps no surprise then that modulating this domain should have an important impact on a cell’s excitability. In fact, previous findings from the Burrone lab were the first to uncover that changes in AIS structure could alter the excitability of neurons to stabilise their overall electrical activity (Grubb & Burrone Nature 365:1070). What is less known is what happens to the unique synapses that form along the AIS, when this region of the axon is modified. “We know very little about these odd axonal synapses, other than they are in the right place to modulate neuronal output by acting directly on the axon initial segment”, says senior author Prof Juan Burrone, “but what happens when the AIS changes was, until now, a mystery”.
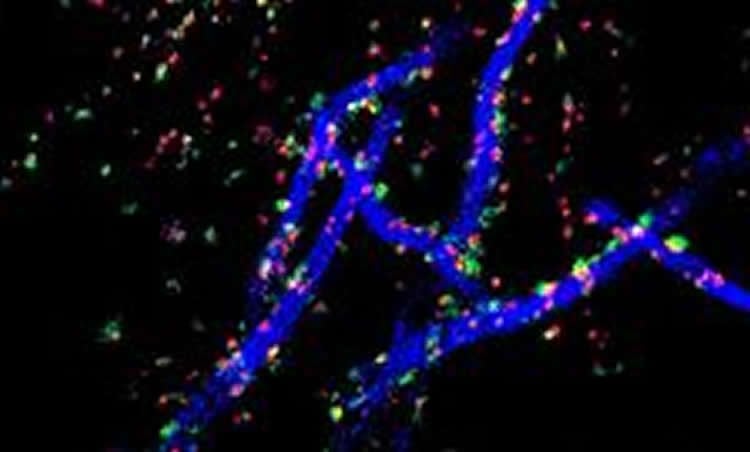
This study shows for the first time that in slices of the hippocampus, a region of the brain known to be important for learning and memory, increases in neuronal activity causes the AIS to move away from the cell body, along the axon, by about half its length. The synapses however stay in place, causing a mismatch between the AIS and the synapses that control it. In collaboration with Dr Daniel Cattaert at the INCIA in Bordeaux, the authors used computational models to study the functional consequences of this arrangement. This approach revealed that the synapses that were left behind, those that lie in the gap between the cell body and the AIS, are particularly important for decreasing neuronal excitability, allowing neurons to remain functional even when under constant stimulation. As Dr Winnie Wefelmeyer, the leading author of this study puts it: “The axon initial segment is like the vocal chord of the neuron: without it, it would be unable to communicate. Changing the position of the AIS relative to the modulating synapses ensures that what the neuron is saying stays meaningful”.
Funding: This study was supported by grants from the Wellcome Trust and the European Research Council.
Source: Andreia Carvalho – Kings College London
Image Credit: The image is credited to Winnie Wefelmeyer/MRC Centre for Developmental Neurobiology
Original Research: Full open access research for “Activity-dependent mismatch between axo-axonic synapses and the axon initial segment controls neuronal output” by Winnie Wefelmeyer, Daniel Cattaert, and Juan Burrone in PNAS. Published online July 20 2015 doi:10.1073/pnas.1502902112
Abstract
Activity-dependent mismatch between axo-axonic synapses and the axon initial segment controls neuronal output
The axon initial segment (AIS) is a structure at the start of the axon with a high density of sodium and potassium channels that defines the site of action potential generation. It has recently been shown that this structure is plastic and can change its position along the axon, as well as its length, in a homeostatic manner. Chronic activity-deprivation paradigms in a chick auditory nucleus lead to a lengthening of the AIS and an increase in neuronal excitability. On the other hand, a long-term increase in activity in dissociated rat hippocampal neurons results in an outward movement of the AIS and a decrease in the cell’s excitability. Here, we investigated whether the AIS is capable of undergoing structural plasticity in rat hippocampal organotypic slices, which retain the diversity of neuronal cell types present at postnatal ages, including chandelier cells. These interneurons exclusively target the AIS of pyramidal neurons and form rows of presynaptic boutons along them. Stimulating individual CA1 pyramidal neurons that express channelrhodopsin-2 for 48 h leads to an outward shift of the AIS. Intriguingly, both the pre- and postsynaptic components of the axo-axonic synapses did not change position after AIS relocation. We used computational modeling to explore the functional consequences of this partial mismatch and found that it allows the GABAergic synapses to strongly oppose action potential generation, and thus downregulate pyramidal cell excitability. We propose that this spatial arrangement is the optimal configuration for a homeostatic response to long-term stimulation.
“Activity-dependent mismatch between axo-axonic synapses and the axon initial segment controls neuronal output” by Winnie Wefelmeyer, Daniel Cattaert, and Juan Burrone in PNAS. Published online July 20 2015 doi:10.1073/pnas.1502902112







