Scientists from Singapore’s Nanyang Technological University (NTU Singapore) and McLean Hospital and Harvard Medical School in the United States have found that existing anti-malaria drugs could be a potential treatment for Parkinson’s disease.
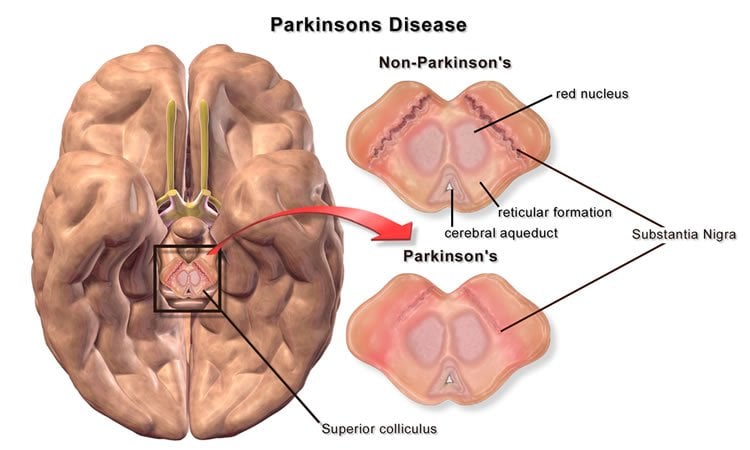
Parkinson’s disease is a degenerative disorder of the central nervous system that causes a person to lose control of motor movements, such as the ability to move his or her hands, arms, and legs.
Parkinson’s disease is one of the most common neurodegenerative conditions in Singapore. It affects three out of every 1,000 persons aged 50 years and above. With an ageing population in Singapore, cases of neurodegenerative diseases are set to rise.
Currently, there is no cure or treatment which can slow down or stop Parkinson’s disease, which affects an estimated 10 million people worldwide.
This groundbreaking research was published recently in Proceedings of the National Academy of Sciences (PNAS) online, a prestigious peer-reviewed scientific journal.
The multi-year research project was a partnership between Professor Kwang-Soo Kim from McLean Hospital and Harvard Medical School in the United States and Associate Professor Yoon Ho Sup from NTU’s School of Biological Sciences.
The team of international scientists had discovered that by activating Nurr1, a class of proteins found in the brain, it protects the brain’s ability to generate dopamine neurons.
Dopamine, commonly known as the chemical in the brain that generates pleasurable feelings, is an important neurotransmitter that affects motor control and movement of muscles in the body.
Parkinson’s disease disrupts the production of dopamine neurons and progressively causes the loss of motor control.
In laboratory tests, the scientists found that by activating Nurr1, the rats which had Parkinson’s disease appeared to improve in their behaviour and showed no signs of suffering from the disease.
Assoc Prof Yoon said the team had screened about 1000 FDA-approved drugs before they found two anti-malaria drugs which worked: Chloroquine and Amodiaquine.
“Our discovery brings hope for the millions of people suffering from Parkinson’s disease, as the drugs that we have found to have worked in the laboratory tests have already been used to treat malaria in patients for decades,” said Assoc Prof Yoon, an expert in drug discovery and design.
“Our research also shows that existing drugs can be repurposed to treat other diseases and once several potential drugs are found, we can redesign them to be more effective in combating their targeted diseases while reducing the side effects.”
Professor Kwang-Soo Kim, a leading expert in Parkinson’s disease, said the current golden standard of treatment is to replenish the patients’ dopamine levels through medication or by using a surgical method to do deep brain stimulation using electric currents.
“However, these pharmacological and surgical treatments address the patient’s symptoms, such as to improve mobility functions in the early stages of the disease, but the treatments cannot slow down or stop the disease process,” Prof Kim explains.
“Backed by various lines of scientific evidence, Nurr1 is known to be a potential drug target to treat Parkinson’s. Despite great efforts from pharmaceutical companies and academia, no one has managed to find a molecule which can directly bind to it and activate it, except for us.”
Both Chloroquine and Amodiaquine are approved by the US Food and Drug Administration and are used treat malaria infections. Chloroquine was used in the late 1940s to early 1950s, until the malaria parasite grew resistant while Amodiaquine is still being used in Africa today.
The scientists are now looking into studying more drugs which can halt and reverse the onset of Parkinson’s disease.
They also aim to design better drugs for the disease by modifying Chloroquine and Amodiaquine. The team hopes to carry out clinical trials with these modified drugs.
Source: Lester Kok – NTU Singapore
Image Credit: The image is credited to BruceBlaus and is licensed CC BY 3.0
Original Research: Abstract for “Nuclear receptor Nurr1 agonists enhance its dual functions and improve behavioral deficits in an animal model of Parkinson’s disease” by Chun-Hyung Kim, Baek-Soo Han, Jisook Moon, Deog-Joong Kim, Joon Shin, Sreekanth Rajan, Quoc Toan Nguyen, Mijin Sohn, Won-Gon Kim, Minjoon Han, Inhye Jeong, Kyoung-Shim Kim, Eun-Hye Lee, Yupeng Tu, Jacqueline L. Naffin-Olivos, Chang-Hwan Park, Dagmar Ringe, Ho Sup Yoon, Gregory A. Petsko, and Kwang-Soo Kim in PNAS. Published only June 29 2015 doi:10.1073/pnas.1509742112
Abstract
Nuclear receptor Nurr1 agonists enhance its dual functions and improve behavioral deficits in an animal model of Parkinson’s disease
Parkinson’s disease (PD), primarily caused by selective degeneration of midbrain dopamine (mDA) neurons, is the most prevalent movement disorder, affecting 1–2% of the global population over the age of 65. Currently available pharmacological treatments are largely symptomatic and lose their efficacy over time with accompanying severe side effects such as dyskinesia. Thus, there is an unmet clinical need to develop mechanism-based and/or disease-modifying treatments. Based on the unique dual role of the nuclear orphan receptor Nurr1 for development and maintenance of mDA neurons and their protection from inflammation-induced death, we hypothesize that Nurr1 can be a molecular target for neuroprotective therapeutic development for PD. Here we show successful identification of Nurr1 agonists sharing an identical chemical scaffold, 4-amino-7-chloroquinoline, suggesting a critical structure–activity relationship. In particular, we found that two antimalarial drugs, amodiaquine and chloroquine stimulate the transcriptional function of Nurr1 through physical interaction with its ligand binding domain (LBD). Remarkably, these compounds were able to enhance the contrasting dual functions of Nurr1 by further increasing transcriptional activation of mDA-specific genes and further enhancing transrepression of neurotoxic proinflammatory gene expression in microglia. Importantly, these compounds significantly improved behavioral deficits in 6-hydroxydopamine lesioned rat model of PD without any detectable signs of dyskinesia-like behavior. These findings offer proof of principle that small molecules targeting the Nurr1 LBD can be used as a mechanism-based and neuroprotective strategy for PD.
“Nuclear receptor Nurr1 agonists enhance its dual functions and improve behavioral deficits in an animal model of Parkinson’s disease” by Chun-Hyung Kim, Baek-Soo Han, Jisook Moon, Deog-Joong Kim, Joon Shin, Sreekanth Rajan, Quoc Toan Nguyen, Mijin Sohn, Won-Gon Kim, Minjoon Han, Inhye Jeong, Kyoung-Shim Kim, Eun-Hye Lee, Yupeng Tu, Jacqueline L. Naffin-Olivos, Chang-Hwan Park, Dagmar Ringe, Ho Sup Yoon, Gregory A. Petsko, and Kwang-Soo Kim in PNAS. Published only June 29 2015 doi:10.1073/pnas.1509742112






