Groundbreaking TAU study tracks precise path of deadly virus to the central nervous system.
Rabies causes acute inflammation of the brain, producing psychosis and violent aggression. The virus, which paralyzes the body’s internal organs, is always deadly for those unable to obtain vaccines in time. Some 55,000 people die from rabies every year.
For the first time, Tel Aviv University scientists have discovered the exact mechanism this killer virus uses to efficiently enter the central nervous system, where it erupts in a toxic explosion of symptoms. The study, published in PLOS Pathogens, was conducted by Dr. Eran Perlson and Shani Gluska of TAU’s Sackler Faculty of Medicine and Sagol School of Neuroscience, in collaboration with the Friedrich Loeffler Institute in Germany.
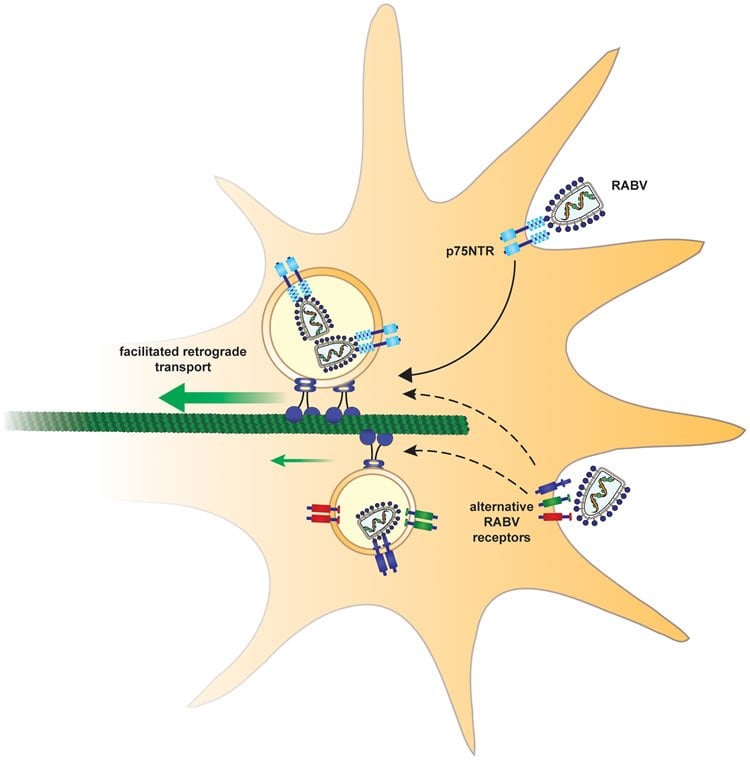
“Rabies not only hijacks the nervous system’s machinery, it also manipulates that machinery to move faster,” said Dr. Perlson. “We have shown that rabies enters a neuron in the peripheral nervous system by binding to a nerve growth factor receptor, responsible for the health of neurons, called p75. The difference is that its transport is very fast, even faster than that of its endogenous ligand, the small molecules that travel regularly along the neuron and keep the neuron healthy.”
Faster than a speeding train
To track the rabies virus in the nervous system, the researchers grew mouse sensory neurons in an observation chamber and used live cell imaging to track the path taken by the virus particles. The researchers “saw” the virus hijack the “train” transporting cell components along a neuron and drove it straight into the spinal cord. Once in the spinal cord, the virus caught the first available train to the brain, where it wrought havoc before speeding through the rest of the body, shutting it down organ by organ.
Nerve cells, or neurons, outside the central nervous system are highly asymmetric. A long protrusion called an axon extends from the cell body to another nerve cell or organ along a specific transmission route. In addition to rapid transmission of electric impulses, axons also transport molecular materials over these distances.
“Axonal transport is a delicate and crucial process for neuronal survival, and when disrupted it can lead to neurodegenerative diseases,” said Dr. Perlson. “Understanding how an organism such as rabies manipulates this machinery may help us in the future to either restore the process or even to manipulate it to our own therapeutic needs.”
Hijacking the hijacker
“A tempting premise is to use this same machinery to introduce drugs or genes into the nervous system,” Dr. Perlson added. By shedding light on how the virus hijacks the transport system in nerve cells to reach its target organ with maximal speed and efficiency, the researchers hope their findings will allow scientists to control the neuronal transport machinery to treat rabies and other neurodegenerative diseases.
Disruptions of the neuron train system also contribute to neurodegenerative diseases, like Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS). According to Dr. Perlson, “An improved understanding of how the neuron train works could lead to new treatments for these disorders as well.”
Contact: George Hunka – American Friends of Tel Aviv University
Source: American Friends of Tel Aviv University press release
Image Source: The image is credited to Perlson et al./PLOS Pathogens and is adapted from the open access research paper
Original Research: Full open access research for “Rabies Virus Hijacks and Accelerates the p75NTR Retrograde Axonal Transport Machinery” by Shani Gluska, Eitan Erez Zahavi, Michael Chein, Tal Gradus, Anja Bauer, Stefan Finke, and Eran Perlson in PLOS Pathogens. Published online August 28 2014 doi:10.1371/journal.ppat.1004348
Rabies Virus Hijacks and Accelerates the p75NTR Retrograde Axonal Transport Machinery
Rabies virus (RABV) is a neurotropic virus that depends on long distance axonal transport in order to reach the central nervous system (CNS). The strategy RABV uses to hijack the cellular transport machinery is still not clear. It is thought that RABV interacts with membrane receptors in order to internalize and exploit the endosomal trafficking pathway, yet this has never been demonstrated directly. The p75 Nerve Growth Factor (NGF) receptor (p75NTR) binds RABV Glycoprotein (RABV-G) with high affinity. However, as p75NTR is not essential for RABV infection, the specific role of this interaction remains in question. Here we used live cell imaging to track RABV entry at nerve terminals and studied its retrograde transport along the axon with and without the p75NTR receptor. First, we found that NGF, an endogenous p75NTR ligand, and RABV, are localized in corresponding domains along nerve tips. RABV and NGF were internalized at similar time frames, suggesting comparable entry machineries. Next, we demonstrated that RABV could internalize together with p75NTR. Characterizing RABV retrograde movement along the axon, we showed the virus is transported in acidic compartments, mostly with p75NTR. Interestingly, RABV is transported faster than NGF, suggesting that RABV not only hijacks the transport machinery but can also manipulate it. Co-transport of RABV and NGF identified two modes of transport, slow and fast, that may represent a differential control of the trafficking machinery by RABV. Finally, we determined that p75NTR-dependent transport of RABV is faster and more directed than p75NTR-independent RABV transport. This fast route to the neuronal cell body is characterized by both an increase in instantaneous velocities and fewer, shorter stops en route. Hence, RABV may employ p75NTR-dependent transport as a fast mechanism to facilitate movement to the CNS.
“Rabies Virus Hijacks and Accelerates the p75NTR Retrograde Axonal Transport Machinery” by Shani Gluska, Eitan Erez Zahavi, Michael Chein, Tal Gradus, Anja Bauer, Stefan Finke, and Eran Perlson in PLOS Pathogens, August 28 2014 doi:10.1371/journal.ppat.1004348.






