The human brain takes up only 2% of our body weight but uses 20% of the body’s energy budget to power the communication between neurons. Stressful conditions put a strain on this energy supply, and disruption of this metabolic state, even briefly, can severely disrupt the brain’s cognitive functions.
While studying the in the C. elegans worm, Yale researchers discovered an emergency “energy generator” that assembles quickly where needed to power synaptic function during times of energy stress.
“Energy demands in neurons are constantly changing, and those demands need to be met locally,” said Daniel Colón-Ramos, associate professor of cell biology and neuroscience and senior author of research published in the journal Neuron.
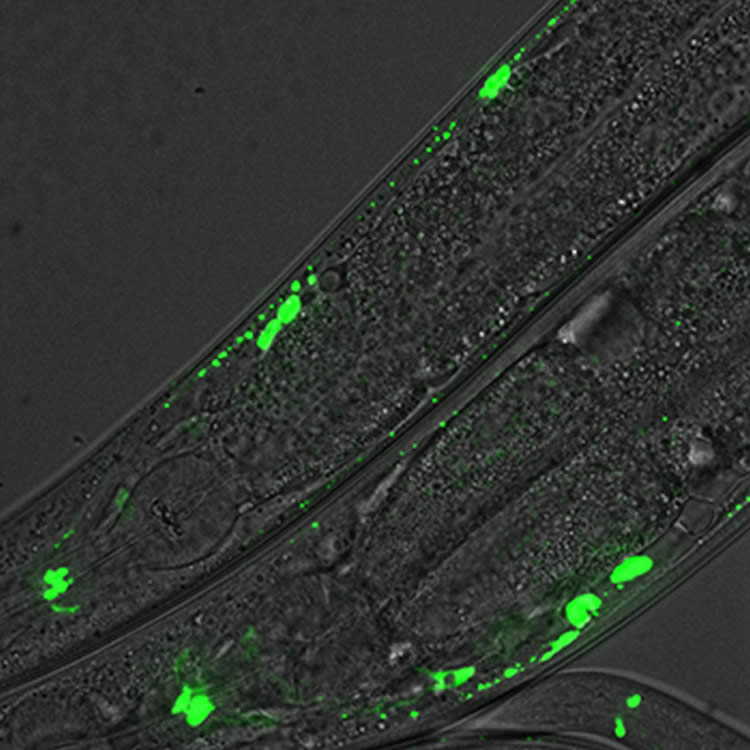
Neuronal activity causes changing energy demands at synapses, the points of contact and communication between neurons. These energy demands must be met locally to sustain synaptic function and brain activity. The primary energy sources are microscopic cellular “power plants” called mitochondria, that shuttle around to cater to local energy demands. However sometimes, even mitochondria can’t keep up with the capricious changes in energy demands observed in neurons.
That’s when glycolytic proteins, ancient enzymes present in all living cells that cooperate in energy production, are mobilized from components throughout the cell to create an emergency generator, the researchers found.
The existence of these generators, called glycolytic metabolons, had been hypothesized for decades but had never been observed in a living organism or in neurons. Unlike the mitochondria, which have all their energy-producing components neatly organized in stable structures, components of the glycolytic metabolon are cytoplasmic, meaning that they are scattered throughout the cell.
“In the nervous system of all animals, information flow in the nervous system comes at a price, and the currency is energy,” Colón-Ramos said. “We think of the glycolytic metabolon as a self-assembling energy generator that allow neurons to produce energy locally, when and where it is needed, to power neural function before the lights go out.”
SoRi Jang of Yale and Jessica C. Nelson, now a postdoctoral fellow at the University of Pennsylvania, are co-first authors of the paper.
Funding: Primary funding for the study came from the National Institutes of Health.
Source: Bill Hathaway – Yale
Image Source: The image is credited to Colon-Ramos laboratory.
Original Research: Abstract for “Glycolytic Enzymes Localize to Synapses under Energy Stress to Support Synaptic Function” by SoRi Jang, Jessica C. Nelson, Eric G. Bend, Lucelenie Rodríguez-Laureano, Felipe G. Tueros, Luis Cartagenova, Katherine Underwood, Erik M. Jorgensen, and Daniel A. Colón-Ramos in Neuron. Published online April 7 2016 doi:10.1016/j.neuron.2016.03.011
Abstract
Glycolytic Enzymes Localize to Synapses under Energy Stress to Support Synaptic Function
Highlights
•A metabolic compartment forms in vivo near synapses to meet local energy demands
•Under energy stress, glycolytic proteins redistribute to form clusters at synapses
•The glycolytic metabolon is needed for the synaptic vesicle cycle
•Disruption of glycolytic metabolon impairs synaptic recovery and affects locomotion
Summary
Changes in neuronal activity create local and transient changes in energy demands at synapses. Here we discover a metabolic compartment that forms in vivo near synapses to meet local energy demands and support synaptic function in Caenorhabditis elegans neurons. Under conditions of energy stress, glycolytic enzymes redistribute from a diffuse localization in the cytoplasm to a punctate localization adjacent to synapses. Glycolytic enzymes colocalize, suggesting the ad hoc formation of a glycolysis compartment, or a “glycolytic metabolon,” that can maintain local levels of ATP. Local formation of the glycolytic metabolon is dependent on presynaptic scaffolding proteins, and disruption of the glycolytic metabolon blocks the synaptic vesicle cycle, impairs synaptic recovery, and affects locomotion. Our studies indicate that under energy stress conditions, energy demands in C. elegans synapses are met locally through the assembly of a glycolytic metabolon to sustain synaptic function and behavior.
“Glycolytic Enzymes Localize to Synapses under Energy Stress to Support Synaptic Function” by SoRi Jang, Jessica C. Nelson, Eric G. Bend, Lucelenie Rodríguez-Laureano, Felipe G. Tueros, Luis Cartagenova, Katherine Underwood, Erik M. Jorgensen, and Daniel A. Colón-Ramos in Neuron. Published online April 7 2016 doi:10.1016/j.neuron.2016.03.011






