Fear response to traumatic or threatening situations helps us evade or escape danger. At the same time fear response is learned in the form of association between stimulus or situation and the presence of a stressor (e.g. physical danger). This association is very powerful and leaves a memory trace that persists for years after a single experience, generating profound structural and functional changes in the brain that can potentially develop into posttraumatic stress and other anxietyrelated disorders.
Fear learning requires only a single experience for the association to be formed and each subsequent exposure to the conditioned stimulus leads to the retrieval of the memory. Both the learning and retrieval of fear memory are characterized by a fearful fightorflight behavioral state associated with a range of unique physiological correlates, such as sweating, tremor, and increased heart rate. Could it be that this very characteristic state of the body is more than just a response to the stressor or conditioned stimulus? Could it be that the fearassociated state of the body rather parallels a characteristic state of the brain that enables learning and memory retrieval?
Animal research provides the means to tackle these questions. In the laboratory research this form of learning is referred to as conditioning and is best represented by classical Pavlovian conditioning in which a neutral tone is repeatedly associated with an aversive stimulus. Fear conditioning gives rise to a lasting memory that in rodents manifests as a stereotypical immobilization response (termed freezing), elicited by the presentation of the tone. Decades of research have identified multiple brain regions that are involved in associative fear learning, including the dorsal medial prefrontal cortex (dmPFC) and the basolateral amygdala (BLA), which have emerged as two structures critical for the learning and expression of fear.
However, while freezing behaviour is widely used as standard measure of fear memory, little is known about the neural mechanisms supporting this behavior and the functional role of the brain state in the learning and retrieval of fear. To fill this important gap, we focused our attention to the study of the neural processes associated with the freezing behavior. To achieve this, we combined electrophysiological recordings of single unit and local field potential activity, as well as optogenetic manipulations of the dmPFC and BLA circuits in freely behaving mice.
In the present study we demonstrate for the first time that freezing behaviour is tightly associated with an internally generated brain state that manifests in sustained 4 Hz oscillatory dynamics in the dmPFCBLA circuits. Furthermore, 4 Hz oscillations accurately predicted onset and termination of the freezing state.
4Hz oscillations provide longrange coupling of the neural activity in dmPFC and BLA, allowing for periods of synchronous coactivation of single neurons, which are believed to be involved in processes of information flow and synaptic plasticity. Using causal analysis we identified that activation of prefrontal neurons precedes that of amygdala neurons within each 4 Hz oscillation cycle, hinting towards the role of prefrontal cortex for controlling the retrieval and expression of fear memories. Using unprecedented optogenetic manipulation, we demonstrated that the artificial induction of 4Hz oscillations in the dmPFC was sufficient to promote freezing behavior and resulted in the formation of longlasting fear memory.
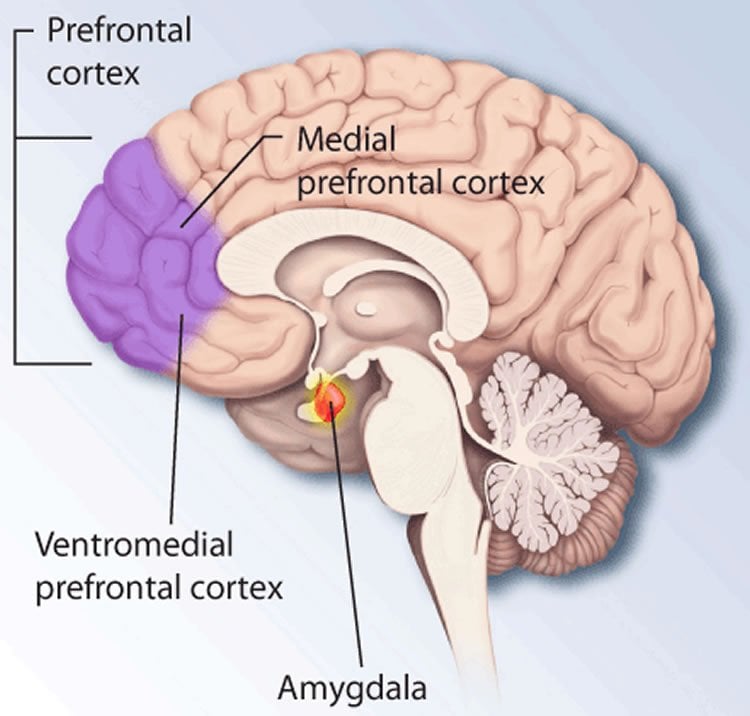
Over the past decades, oscillations have emerged as an important mechanism for the organization of collective neural activity and are implicated in both the physiological and pathological functioning of the brain. Exploratory state is associated with distinct internal dynamics known as theta (~8 hz) oscillations, while deep sleep state is characterized by slow (~1Hz) oscillations. Our results identify a novel internally generated brain state characterized by 4Hz oscillations and associated with the expression of fear that provides a new framework for the study of the neural mechanisms involved in fear memory formation, retrieval, and expression.
Together, our results unravel a physiological signature of fear memory within dmPFCBLA networks and further suggest that blocking oscillations in this circuit might represent a potential therapeutic strategy for pathological conditions such as anxiety disorders.
This article was submitted directly to NeuroscienceNews.com by Nikolas Karalis. We would like to thank Nikolas for providing us with this research article.
Source: Nikolas Karalis – Ludwig-Maximilians Universität München
Image Credit: The image is credited to NIMH and is in the public domain
Original Research: Abstract for “4-Hz oscillations synchronize prefrontal–amygdala circuits during fear behavior” by Nikolaos Karalis, Cyril Dejean, Fabrice Chaudun, Suzana Khoder, Robert R Rozeske, Hélène Wurtz, Sophie Bagur, Karim Benchenane, Anton Sirota, Julien Courtin and Cyril Herry in Nature Neuroscience. Published online February 15 2016 doi:10.1038/nn.4251
Abstract
4-Hz oscillations synchronize prefrontal–amygdala circuits during fear behavior
Fear expression relies on the coordinated activity of prefrontal and amygdala circuits, yet the mechanisms allowing long-range network synchronization during fear remain unknown. Using a combination of extracellular recordings, pharmacological and optogenetic manipulations, we found that freezing, a behavioral expression of fear, temporally coincided with the development of sustained, internally generated 4-Hz oscillations in prefrontal–amygdala circuits. 4-Hz oscillations predict freezing onset and offset and synchronize prefrontal–amygdala circuits. Optogenetic induction of prefrontal 4-Hz oscillations coordinates prefrontal–amygdala activity and elicits fear behavior. These results unravel a sustained oscillatory mechanism mediating prefrontal–amygdala coupling during fear behavior.
“4-Hz oscillations synchronize prefrontal–amygdala circuits during fear behavior” by Nikolaos Karalis, Cyril Dejean, Fabrice Chaudun, Suzana Khoder, Robert R Rozeske, Hélène Wurtz, Sophie Bagur, Karim Benchenane, Anton Sirota, Julien Courtin and Cyril Herry in Nature Neuroscience. Published online February 15 2016 doi:10.1038/nn.4251