Summary: Abnormal activity involving the globus pallidus may be responsible for movement dysfunction in Parkinson’s disease, a new study reports.
Source: Northwestern University.
The brain’s own mechanisms for dealing with the loss of dopamine neurons in Parkinson’s disease may be a source of the disorder’s abnormal movement, according to a Northwestern Medicine study published in Neuron.
The study suggests the loss of dopamine may cause the brain to rewire in a maladaptive manner, contributing to impaired movement in Parkinson’s disease. These findings also suggest that there are fundamental problems with scientists’ traditional model of Parkinson’s disease, said senior author Mark Bevan, PhD, professor of Physiology at Northwestern University Feinberg School of Medicine.
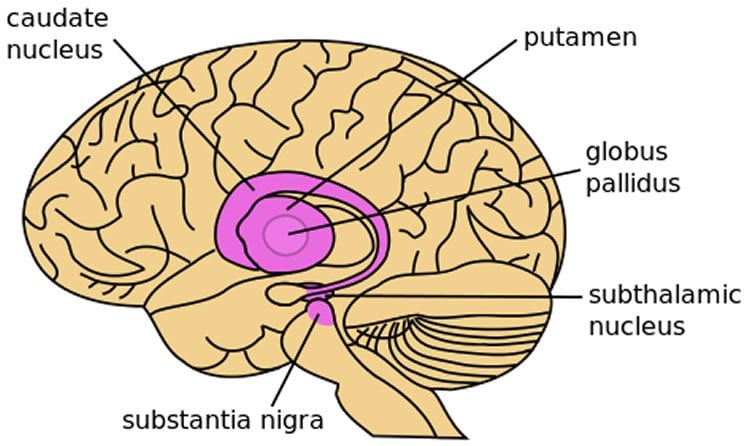
The prevailing consensus was that excessive patterning of the subthalamic nucleus (STN), a component of the basal ganglia, by the cerebral cortex was linked to the symptomatic expression of Parkinson’s disease, including muscle rigidity and slowness of movement, according to Bevan.
“When one saw a burst of activity in the cortex that was consistently followed by an abnormal burst of activity in the STN, scientists assumed that the direct connection between the two was responsible,” Bevan said.
Thus, Bevan and his colleagues, including lead author Hong-Yuan Chu, PhD, a post-doctoral fellow in the Bevan Lab, expected to see transmission in the cortex-to-STN pathway increase as dopamine levels dropped. Instead, they found the opposite: the strength of the pathway decreased massively.
“Like most scientists who come across something unexpected, we thought we’d done something wrong,” Bevan said. “So, we used multiple, complementary approaches but everything pointed to the same conclusion.”
Further investigation suggested abnormal activity in a more indirect pathway from the cortex to the STN, involving the globus pallidus, was responsible. Abnormal activity in the indirect pathway leaves the STN vulnerable to excessive excitation, triggering compensatory plasticity that ultimately proved to be harmful, according to the study.
When the scientists prevented this maladaptive plasticity in late-stage Parkinson’s models, they found the symptoms improved, pointing to a link between compensation and motor dysfunction.
“According to the classic model, these adaptations should be homeostatic and preserve STN function,” Bevan said. “Preventing them should make the symptoms much worse — but it made them better instead.”
While the compensatory mechanisms may initially keep the brain operating normally under conditions of moderate dopamine neuron loss, as the disease progresses and more dopamine neurons die, the adaptations may become so extreme that they impair movement, according to the study.
These results suggest that there are fundamental flaws in our traditional understanding of brain dysfunction in Parkinson’s disease, Bevan said.
For Bevan, the unexpected results in this study served as a reminder that scientists must remain open-minded.
“It’s easy to be emotional and cling to your hypothesis,” Bevan said. “You have to be dispassionate, open-minded, and look at the data — if the data is not consistent with the hypothesis then you have to reject it and come up with a new one.”
Funding: This study was funded by the National Institutes of Health’s National Institute of Neurological Disorders and Stroke grants 2R37 NS041280, P50 NS047085, 5T32 NS041234, and F31 NS090845. Confocal imaging work was performed at the Northwestern University Center for Advanced Microscopy, which was supported by National Cancer Institute Cancer Center Support grant P30 CA060553.
Source: Marla Paul – Northwestern University
Image Source: NeuroscienceNews.com image is in the public domain.
Original Research: Abstract for “Loss of Hyperdirect Pathway Cortico-Subthalamic Inputs Following Degeneration of Midbrain Dopamine Neurons” by Hong-Yuan Chu, Eileen L. McIver, Ryan F. Kovaleski, Jeremy F. Atherton, and Mark D. Bevan in Neuron. Published online September 13 2017 doi:10.1016/j.neuron.2017.08.038
[cbtabs][cbtab title=”MLA”]Northwestern University “Brain Rewiring in Parkinson’s Disease May Contribute to Abnormal Movement.” NeuroscienceNews. NeuroscienceNews, 16 September 2017.
<https://neurosciencenews.com/parkinsons-movement-brain-7503/>.[/cbtab][cbtab title=”APA”]Northwestern University (2017, September 16). Brain Rewiring in Parkinson’s Disease May Contribute to Abnormal Movement. NeuroscienceNews. Retrieved September 16, 2017 from https://neurosciencenews.com/parkinsons-movement-brain-7503/[/cbtab][cbtab title=”Chicago”]Northwestern University “Brain Rewiring in Parkinson’s Disease May Contribute to Abnormal Movement.” https://neurosciencenews.com/parkinsons-movement-brain-7503/ (accessed September 16, 2017).[/cbtab][/cbtabs]
Abstract
Loss of Hyperdirect Pathway Cortico-Subthalamic Inputs Following Degeneration of Midbrain Dopamine Neurons
Highlights
•Cortico-STN synaptic transmission is reduced by 50%–75% in PD mice
•Increased striato-pallidal transmission triggers cortico-STN input loss
•Cortico-STN input loss in PD mice is NMDAR dependent
•Reduction of STN plasticity is therapeutic in PD mice
Summary
The motor symptoms of Parkinson’s disease (PD) are linked to abnormally correlated and coherent activity in the cortex and subthalamic nucleus (STN). However, in parkinsonian mice we found that cortico-STN transmission strength had diminished by 50%–75% through loss of axo-dendritic and axo-spinous synapses, was incapable of long-term potentiation, and less effectively patterned STN activity. Optogenetic, chemogenetic, genetic, and pharmacological interrogation suggested that downregulation of cortico-STN transmission in PD mice was triggered by increased striato-pallidal transmission, leading to disinhibition of the STN and increased activation of STN NMDA receptors. Knockdown of STN NMDA receptors, which also suppresses proliferation of GABAergic pallido-STN inputs in PD mice, reduced loss of cortico-STN transmission and patterning and improved motor function. Together, the data suggest that loss of dopamine triggers a maladaptive shift in the balance of synaptic excitation and inhibition in the STN, which contributes to parkinsonian activity and motor dysfunction.
“Loss of Hyperdirect Pathway Cortico-Subthalamic Inputs Following Degeneration of Midbrain Dopamine Neurons” by Hong-Yuan Chu, Eileen L. McIver, Ryan F. Kovaleski, Jeremy F. Atherton, and Mark D. Bevan in Neuron. Published online September 13 2017 doi:10.1016/j.neuron.2017.08.038







