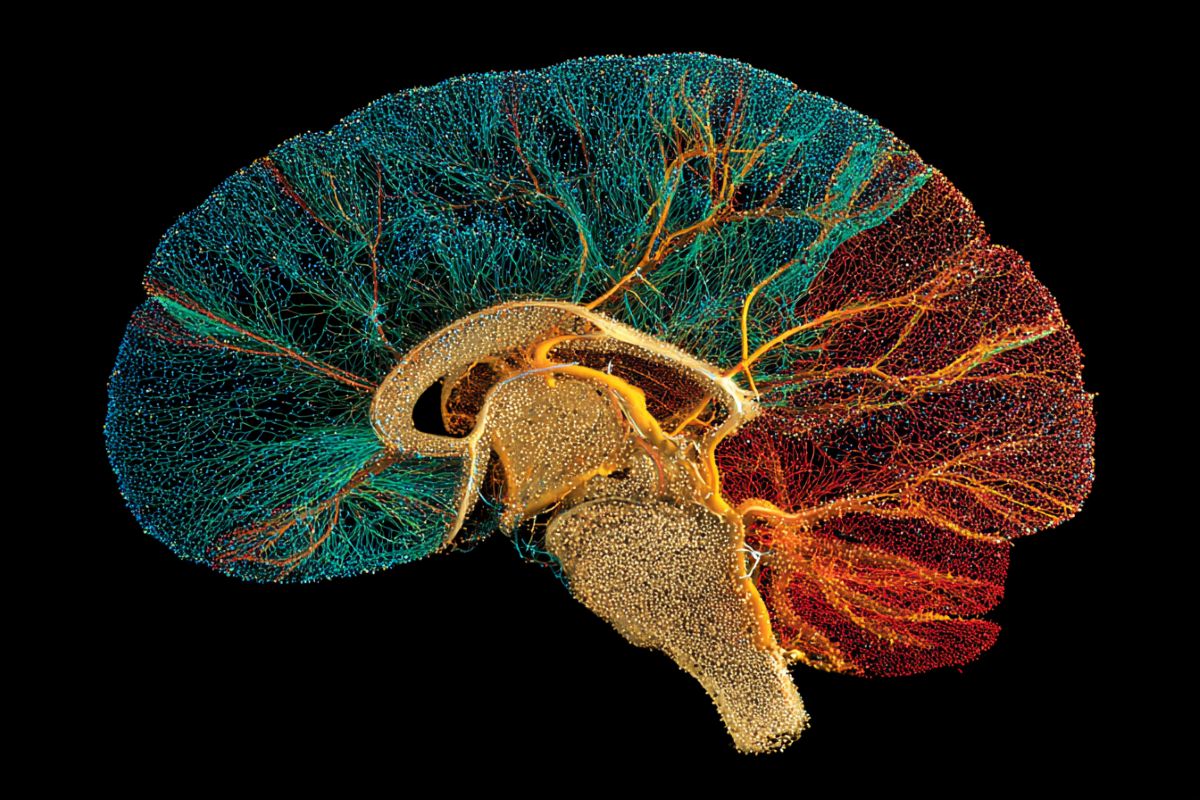
Summary: Using advanced 3D imaging, light-sheet microscopy, and AI, scientists have created the most precise maps to date of oligodendrocytes—the brain cells responsible for making myelin. Myelin acts as a protective “insulation” for nerve fibers, allowing electrical signals to travel rapidly through the brain.
These maps track over 10 million cells across the lifespan of a mouse, providing unprecedented resolution of gray matter—an area notoriously difficult to image. This “forest map” of the brain’s ecosystem offers vital clues into why myelin fails in diseases like Multiple Sclerosis, Alzheimer’s, and other memory-related disorders.
Key Facts
- Massive Scale: The study mapped the exact location of more than 10 million oligodendrocytes in the mouse brain.
- Gray Matter Breakthrough: Unlike MRI, which struggles with gray matter, these new maps provide high-resolution coverage of where neurons control movement and function.
- Lifespan Tracking: The maps show that while the brain steadily adds oligodendrocytes from age two months to two years, the rate of growth is remarkably consistent and region-specific.
- Sensory Speed: Brain regions receiving direct sensory input (sight, sound, touch) have three times more oligodendrocytes than the motor cortex, likely to ensure lightning-fast processing.
- Alzheimer’s Vulnerability: In Alzheimer’s models, myelin damage was found not just near dense protein plaques but also in areas with diffuse plaques, explaining why white matter dysfunction is so common in the disease.
Source: Johns Hopkins University
Johns Hopkins scientists say they have used 3D imaging, special microscopes and artificial intelligence (AI) programs to construct new maps of mouse brains showing a precise location of more than 10 million cells called oligodendrocytes.
These cells form myelin, a protective sleeve around nerve cell axons, which speeds transmission of electrical signals and support brain health.
Published online Feb. 18 in Cell and funded by the National Institutes of Health, the maps not only paint a whole-brain picture of how myelin content varies between brain circuits, but also provide insights into how the loss of such cells impacts human diseases such as multiple sclerosis, Alzheimer’s disease and other disorders that affect learning, memory, sensory ability and movement, say the researchers.
Although mouse and human brains are not the same, they share many characteristics and most biological processes.
“Our study identifies not only the location of oligodendrocytes in the brain, but also integrates information about gene expression and the structural features of neurons,” says Dwight Bergles, Ph.D., the Diana Sylvestre and Charles Homcy Professor in the Department of Neuroscience at the Johns Hopkins University School of Medicine.
“It’s like mapping the location of all the trees in a forest, but also adding information about soil quality, weather and geology to understand the forest ecosystem.”
The Johns Hopkins-created maps provide higher resolution and better coverage of gray matter than previously published maps, they say. Myelin in these areas is harder to see using techniques such as MRI. Gray matter houses most of the neurons in the brain and controls movement and other functions.
“Because myelin can speed communication between neurons, these maps of regional differences in myelin patterning may help us understand how different parts of the brain accomplish different tasks,” says Bergles.
Oligodendrocytes are found in nearly every area of the brain, even though myelin is more prevalent in white matter, which serves as the main highway for neural circuits connecting different regions of the brain.
For the new mapping project, Bergles’ team, including first author Yu Kang T. Xu, a Ph.D. student and Kavli Neuroscience Discovery Institute fellow, collaborated with biomedical engineers and computer scientists.
They developed a novel pipeline involving tissue clearing, which removes fatty deposits that make it hard to see deep in the brain, along with a fast type of imaging called light-sheet microscopy to rapidly scan through all brain structures.
To catalog over 10 million cells per mouse brain in various conditions and timespans, the scientists needed help from machine learning, a technology that teaches computers how to accurately perform tasks — in this case, to automatically search through images and identify each oligodendrocyte, then reconstruct brainwide maps, one image at a time.
Each map charted positions of oligodendrocytes at certain times over the mouse lifespan, from age two months to two years. With age, animals steadily acquired more oligodendrocytes, but the rate of new oligodendrocyte and myelin formation varied dramatically between different brain regions.
Areas that had slow addition at first continued to add oligodendrocytes slowly later in life — they didn’t suddenly catch up or show dramatic variability — suggesting that this patterning reflects a fairly rigid developmental program.
“It will be interesting to use this approach to see how different life experiences, such as stress, social interaction, and learning affect these patterns,” Bergles says.
Oligodendrocyte and myelin formation were very prolonged in areas of the mammalian brain, such as the hippocampus, which are key to the formation and storage of learning and memory.
They also found that brain regions that received direct sensory input had three times more oligodendrocytes than other areas such as the primary motor cortex. This difference may reflect the brain’s need to have myelin-wrapped neurons with faster transmission located in areas that need to process sensory information — touch, sound and sight, for example — very quickly.
In mice exposed to chemicals that destroy oligodendrocytes and myelin, the scientists identified regions of higher vulnerability and greater resilience, which may yield clues to preserving myelin in diseases such as multiple sclerosis.
Finally, in a mouse model of Alzheimer’s disease, the team found that myelin was not only damaged in areas near amyloid-beta plaques called dense core plaques (a tangle of misfolded proteins that are a hallmark of Alzheimer’s disease), but also in white matter regions with only diffuse plaques. This increased vulnerability may explain why oligodendrocyte dysfunction is prevalent in this disease, says Bergles.
These newly published oligodendrocyte maps can be explored free of charge by other scientists, says Bergles, with hopes such use will hasten new discoveries.
Funding: This work is supported by the National Institutes of Health (AG072305, NS041435, 32GM136577), Chan Zuckerberg Initiative, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation and the Kavli Neuroscience Discovery Institute.
Key Questions Answered:
A: Think of them as the “electricians” of the brain. They wrap nerve fibers in a fatty sleeve called myelin. Without this insulation, the electrical signals in your brain would leak out or slow down, much like a frayed wire short-circuiting.
A: To understand the ecosystem. By knowing exactly where these “insulators” are located, scientists can see which brain circuits are the fastest and which are the most vulnerable to aging or disease. It’s the difference between having a blurry photo of a city and a GPS map that shows every individual house.
A: MS is caused by the immune system attacking myelin. These maps identified specific regions of the brain that are more “resilient” or “vulnerable” to myelin loss, which could lead to new treatments that protect those weak spots or encourage repair.
Editorial Notes:
- This article was edited by a Neuroscience News editor.
- Journal paper reviewed in full.
- Additional context added by our staff.
About this neuroscience research news
Author: Vanessa Wasta
Source: Johns Hopkins University
Contact: Vanessa Wasta – Johns Hopkins University
Image: The image is credited to Neuroscience News
Original Research: Open access.
“Brain-wide mapping of oligodendrocyte organization, oligodendrogenesis, and myelin injury” by Yu Kang T. Xu, Abigail Bush, Ephraim Musheyev, Jacob Umans, Lingzi Zhang, Anya A. Kim, Sen Zhang, Jaime Eugenin von Bernhardi, Yuqing Yan, Jeremias Sulam, and Dwight E. Bergles. Cell
DOI:10.1016/j.cell.2026.01.025
Abstract
Brain-wide mapping of oligodendrocyte organization, oligodendrogenesis, and myelin injury
Insulating sheaths of myelin accelerate neuronal communication in the mammalian brain. Oligodendrocytes that produce myelin are generated throughout life to gradually increase myelin coverage, but these dynamics have not been defined brain-wide across the lifespan.
We developed a cellular mapping pipeline involving tissue clearing, lightsheet microscopy, and AI-assisted analysis to identify the precise location of millions of oligodendrocytes and assess regional myelin density in the mouse brain.
These atlases revealed the diversity of oligodendrocyte patterning, which was consistent between brain hemispheres, individuals, and sexes but displayed both age- and region-specific differences.
Integration of these atlases with transcriptomic and ultrastructural datasets highlighted underlying mechanisms that may control this patterning. In models of demyelination and disease, we identified regions of enhanced oligodendrocyte resilience and vulnerability and white matter injury near β-amyloid plaques, demonstrating the utility of this pipeline for defining brain-wide oligodendrocyte dynamics in both health and disease.