Summary: Max Planck researchers have made a groundbreaking discovery that helps explain why people with Parkinson’s often report an impaired sense of smell. They discovered the total volume of glomeruli in the olfactory bulb of Parkinson’s patients is only half of that found in people without the disease.
Source: Max Planck Institute.
The first symptom of Parkinson’s disease is often an impaired sense of smell. This neurodegenerative disease primarily causes irreparable damage to nerve cells in a brain area involved in movement control. How it affects the olfactory system has been unclear. Researchers at the Max Planck Research Unit for Neurogenetics in Frankfurt and the University of Auckland in New Zealand have now carried out a study comparing the olfactory bulbs of individuals with and without Parkinson’s disease. The researchers found that the total volume occupied by the functional units in the olfactory bulb – the so-called glomeruli – is in Parkinson’s cases only half that in normal individuals. Moreover, the distribution of the glomeruli within the olfactory bulb is altered in Parkinson’s cases.
Nine out of ten patients with Parkinson’s disease suffer from defects of the sense of smell in the early stages of the disease – often years before the appearance of the motor symptoms that are characteristic of the disease. The motor symptoms are caused by a loss of nerve cells in the region of the substantia nigra in the brain that is responsible for controlling movement. What causes this cell death has not yet been fully clarified, but a key role appears to be played by Lewy bodies. These are inclusions, inside the cells, that contain a misfolded, defective version of the alpha-synuclein protein. Lewy bodies are found in the olfactory bulb before they appear in the substantia nigra.
The so-called olfactory vector hypothesis for Parkinson’s disease proposes that environmental factors, such as viruses, heavy metals or pesticides, are risk factors or even causes of the condition. No other sensory system than the olfactory system is in such close contact with the external environment – the inhaled air. The hypothesis posits that the disease-causing agent is introduced from the nasal cavity into the olfactory bulb, where Parkinson’s disease is triggered and gradually spreads through other parts of the brain.
Intact tissue samples required
The human olfactory bulb remains poorly studied. Research on this brain structure depends critically on the availability of pristine samples, which are typically procured post mortem, from brain donors. The Neurological Foundation of New Zealand Douglas Human Brain Bank in Auckland, New Zealand works closely with families of patients suffering from neurodegenerative diseases to ensure ethical and effective collection of post mortem brain samples from diseased and non-diseased cases. The precarious location of the olfactory bulb below the bulk of the brain and the many axons that connect it to the olfactory mucosa mean that special efforts must be made to protect the morphology of the olfactory bulb when collecting the samples.
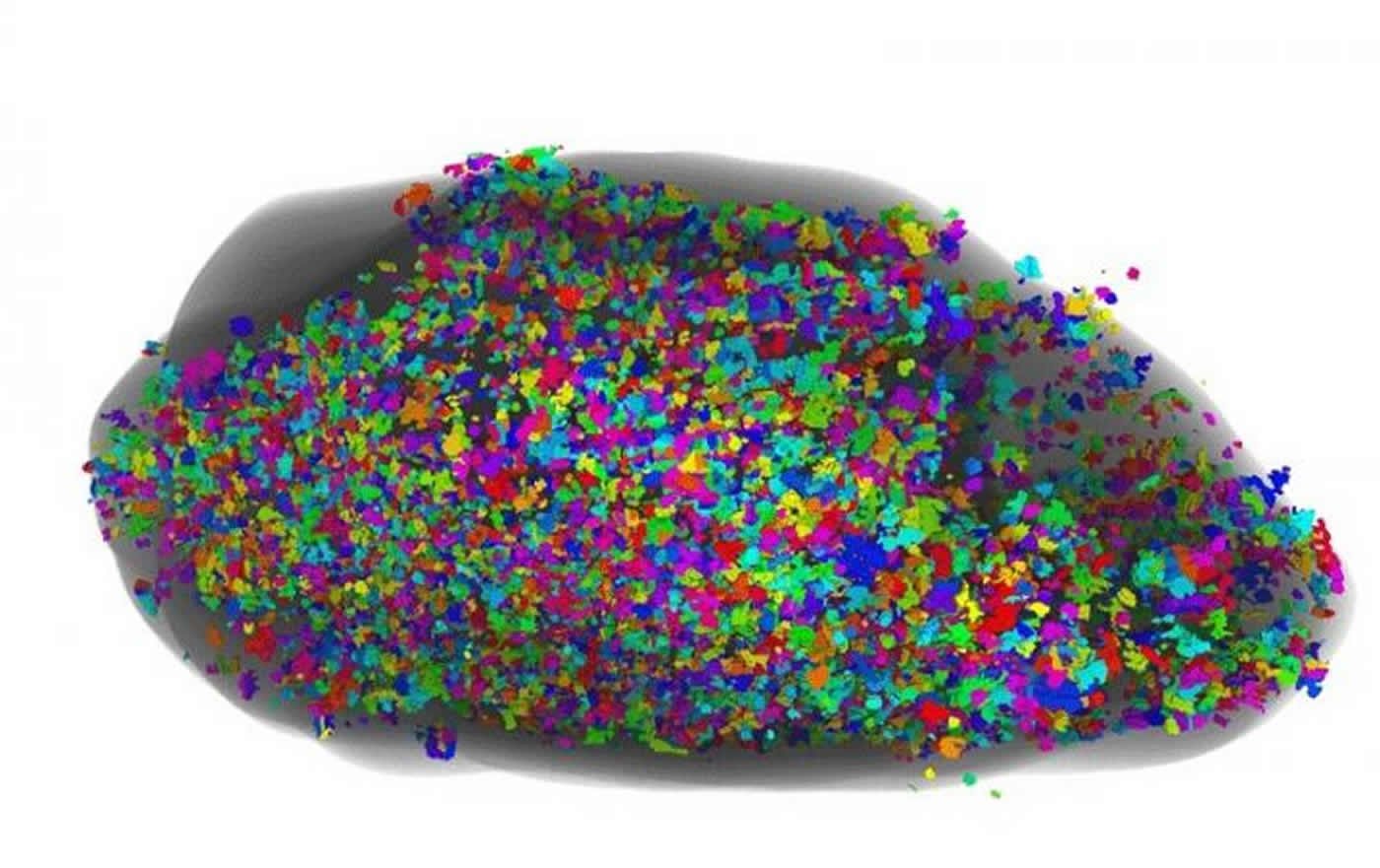
This is a ventral view of glomeruli in a human olfactory bulb. Single glomeruli are represented in confetti-like colours. Credit: Max Planck Research Unit for Neurogenetics.
The New Zealand-based researchers were able to collect olfactory bulbs fit for an in-depth quantitative study. In a globe-spanning project, the researchers processed the post mortem olfactory bulbs chemically, cut ten-micrometer thin sections throughout its entire length, and stained the sections with fluorescently labeled antibodies. The labeled sections were then scanned in Frankfurt, and the images reconstructed in 3D allowing for quantitative whole-olfactory bulb analyses.
New quantitive parameter
As glomeruli of the human olfactory bulb are difficult to count unambiguously, the researchers came up with a new, quantitative parameter: the global glomerular voxel volume. This quantity is the sum of the volume of all glomeruli. These are formed by the coalescence of axons of olfactory sensory neurons making synapses with olfactory bulb neurons. Having defined this new parameter, the researchers compared the values between olfactory bulbs from normal and Parkinson’s disease cases, and found that it was reduced by more than half. Whether the decrease is the result of Parkinson’s disease cases having fewer or smaller glomeruli, or is due to a combination of these two effects, remains to be seen.
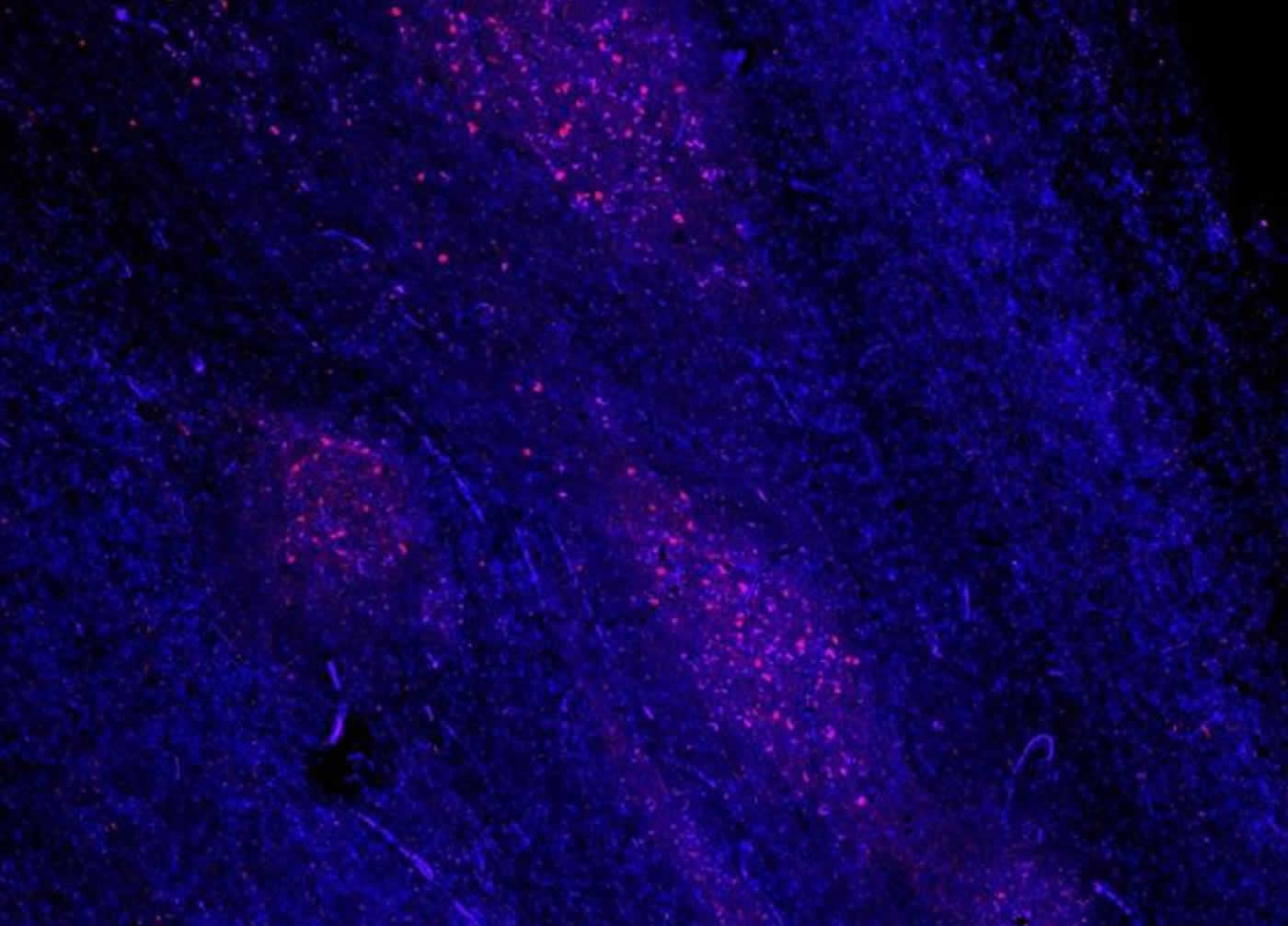
Lewy bodies and Lewy neurites are filled with misfolded alpha-synuclein and are hallmark pathologies of brain tissue in Parkinson’s disease. The nuclei of olfactory bulb cells are visualized with a blue-fluorescent dye. The abnormal alpha-synuclein is visualized in red fluorescence by staining with a specific antibody. NeuroscienceNews.com image is credited to Max Planck Research Unit for Neurogenetics.
In addition, the distribution of the glomeruli was altered. The olfactory bulbs of normal cases had 70 percent of their glomerular component in the bottom half of the olfactory bulb, but the olfactory bulbs of Parkinson’s disease cases contained only 44 percent in the bottom half. “The preferential deficit of the glomerular component in the bottom half of the olfactory bulb, close to the olfactory mucosa, is consistent with the olfactory vector hypothesis of Parkinson’s disease”, states Peter Mombaerts, M.D., Ph.D., director of the Max Planck Research Unit for Neurogenetics. The scientists also discovered that the greater the number of Lewy bodies with aggregated alpha synuclein, the smaller the glomerular component of the olfactory bulb. “This relationship could be an indication that the Lewy bodies are the cause of the reduction in glomerular volume,” explains Dr. Bolek Zapiec, first author of the paper.
The question now is which type of neurons in the olfactory bulb is affected first or foremost in Parkinson’s disease. Next the researchers would like to identify the neurons in the olfactory bulb that are the most vulnerable.
Source: Peter Mombaerts – Max Planck Institute
Image Source: NeuroscienceNews.com images are credited to Max Planck Research Unit for Neurogenetics.
Original Research: Full open access research for “A ventral glomerular deficit in Parkinson’s disease revealed by whole olfactory bulb reconstruction” by Bolek Zapiec, Birger V. Dieriks, Sheryl Tan, Richard L. M. Faull, Peter Mombaerts, and Maurice A. Curtis in Brain. Published online September 3 2017 doi:10.1093/brain/awx208
[cbtabs][cbtab title=”MLA”]Max Planck Institute “Parkinson’s Involves Degeneration of the Olfactory System.” NeuroscienceNews. NeuroscienceNews, 28 September 2017.
<https://neurosciencenews.com/olfactory-system-parkinsons-7611/>.[/cbtab][cbtab title=”APA”]Max Planck Institute (2017, September 28). Parkinson’s Involves Degeneration of the Olfactory System. NeuroscienceNews. Retrieved September 28, 2017 from https://neurosciencenews.com/olfactory-system-parkinsons-7611/[/cbtab][cbtab title=”Chicago”]Max Planck Institute “Parkinson’s Involves Degeneration of the Olfactory System.” https://neurosciencenews.com/olfactory-system-parkinsons-7611/ (accessed September 28, 2017).[/cbtab][/cbtabs]
Abstract
A ventral glomerular deficit in Parkinson’s disease revealed by whole olfactory bulb reconstruction
Olfactory dysfunction is common in Parkinson’s disease and is an early symptom, but its pathogenesis remains poorly understood. Hindering progress in our mechanistic understanding of olfactory dysfunction in Parkinson’s disease is the paucity of literature about the human olfactory bulb, both from normal and Parkinson’s disease cases. Qualitatively it is well established that the neat arrangement of the glomerular array seen in the mouse olfactory bulb is missing in humans. But rigorous quantitative approaches to describe and compare the thousands of glomeruli in the human olfactory bulb are not available. Here we report a quantitative approach to describe the glomerular component of the human olfactory bulb, and its application to draw statistical comparisons between olfactory bulbs from normal and Parkinson’s disease cases. We subjected horizontal 10 µm sections of olfactory bulbs from six normal and five Parkinson’s disease cases to fluorescence immunohistochemistry with antibodies against vesicular glutamate transporter-2 and neural cell adhesion molecule. We scanned the immunostained sections with a fluorescence slide scanner, segmented the glomeruli, and generated 3D reconstructions of whole olfactory bulbs. We document the occurrence of atypical glomerular morphologies and glomerular-like structures deep in the olfactory bulb, both in normal and Parkinson’s disease cases. We define a novel and objective parameter: the global glomerular voxel volume, which is the total volume of all voxels that are classified immunohistochemically as glomerular. We find that the global glomerular voxel volume in Parkinson’s disease cases is half that of normal cases. The distribution of glomerular voxels along the dorsal-ventral dimension of the olfactory bulb in these series of horizontal sections is significantly altered in Parkinson’s disease cases: whereas most glomerular voxels reside within the ventral half of olfactory bulbs from normal cases, glomerular voxels are more evenly spread among the ventral and dorsal halves of olfactory bulbs from Parkinson’s disease cases. These quantitative whole-olfactory bulb analyses indicate a predominantly ventral deficit in the glomerular component in Parkinson’s disease, consistent with the olfactory vector hypothesis for the pathogenesis of this neurodegenerative disease. The distribution of serine 129-phosphorylated α-synuclein immunoreactive voxels correlates with that of glomerular voxels. The higher the serine 129-phosphorylated α-synuclein load of an olfactory bulb from a Parkinson’s disease case, the lower the global glomerular voxel volume. Our rigorous quantitative approach to the whole olfactory bulb will help understand the anatomy and histology of the normal human olfactory bulb and its pathological alterations in Parkinson’s disease.
“A ventral glomerular deficit in Parkinson’s disease revealed by whole olfactory bulb reconstruction” by Bolek Zapiec, Birger V. Dieriks, Sheryl Tan, Richard L. M. Faull, Peter Mombaerts, and Maurice A. Curtis in Brain. Published online September 3 2017 doi:10.1093/brain/awx208






