Summary: Researchers report nitric oxide plays a key role in regulating neural function. The findings shed new light on how neurodegeneration may occur.
Source: University of Leicester.
Researchers from the University of Leicester have shed new light on how neurons in the brain communicate with one another. This could potentially help in our understanding of how and why a range of neurodegenerative diseases occur.
The team, led by Dr Joern Steinert from the MRC Toxicology Unit at the University of Leicester, has found that the important molecule present in the whole animal kingdom — including the human body — called nitric oxide, plays a vital role in regulating the functions of neurons not only periphery but also in the brain, helping to decipher the ‘mosaic’ of knowledge about how our brain communicates.
Nitric oxide is a signalling molecule involved in many physiological and pathological processes which helps in dilating blood vessels, raising blood supply and lowering blood pressure.
The new research, which is funded by the Medical Research Council and published in the journal PLOS Biology, has found that nitric oxide also regulates the functions of neurons via a modulation of a signalling step at a synapse – the point where two neurons connect and neurotransmitters are released.
This regulation changes the position of the protein — called complexin — within a synapse and regulates the amount of the neurotransmitter which is released.
“We showed for the first time that this complexin protein can be regulated or modified so that it is now able to adjust the function of a synapse and eventually the neuron,” says Dr Steinert. “This would have bigger impacts on the global function of the brain which has to be constantly regulated and adjusted to changes in demand. Implications are also related to neurological disease, in which this exact signalling might go wrong and leaves the neuron unable to function.
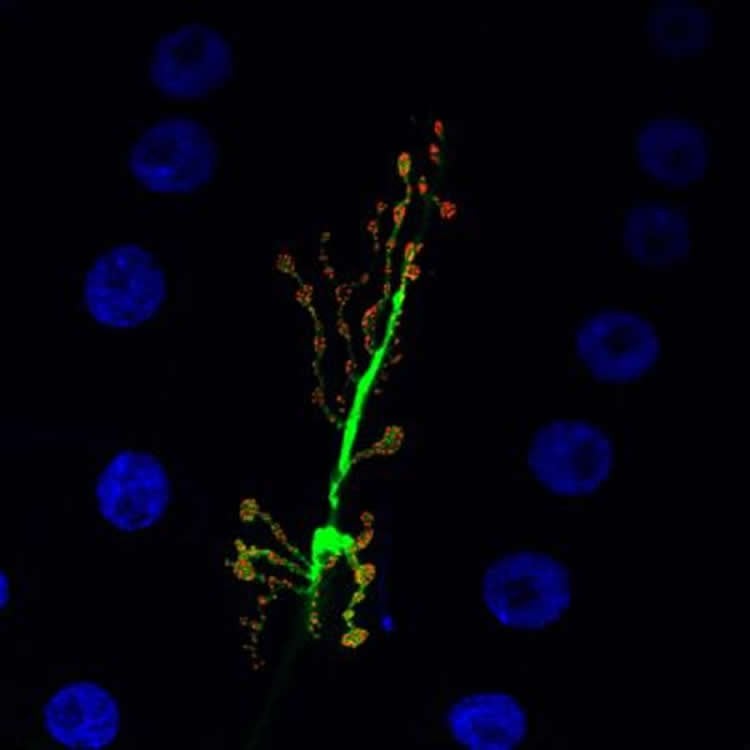
“This research can also help to better understand neurological conditions, such as seen in many neurodegenerative diseases. If the pathways which we characterised go wrong, it can easily disrupt whole brain function and leading to neuronal death.”
The team investigated the neural pathway in the fruit fly Drosophila melanogaster. They predominately detected changes in neuronal function — or the electrical firing of a single neuron.
The researchers then used genetic methods to express certain proteins of interest at in specific neurons, using various methods to visualise proteins and molecules within neurons in order to detect changes in their position and function within a neuron.
Funding: Medical Research Council funded this study.
Source: Joern Steinert – University of Leicester
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to University of Leicester.
Original Research: Open access research for “Nitric oxide-mediated posttranslational modifications control neurotransmitter release by modulating complexin farnesylation and enhancing its clamping ability” by Susan W. Robinson, Julie-Myrtille Bourgognon, Jereme G. Spiers, Carlo Breda, Susanna Campesan, Adrian Butcher, Giovanna R. Mallucci, David Dinsdale, Nobuhiro Morone, Raj Mistry, Tim M. Smith, Maria Guerra-Martin, R. A. John Challiss, Flaviano Giorgini, and Joern R. Steinert in PLOS Biology. Published April 9 2018.
doi:10.1371/journal.pbio.2003611
[cbtabs][cbtab title=”MLA”]University of Leicester “Discovery Sheds Light on How Neurodegenerative Diseases May Occur.” NeuroscienceNews. NeuroscienceNews, 10 April 2018.
<https://neurosciencenews.com/nitric-oxide-neurodegeneration-8753/>.[/cbtab][cbtab title=”APA”]University of Leicester (2018, April 10). Discovery Sheds Light on How Neurodegenerative Diseases May Occur. NeuroscienceNews. Retrieved April 10, 2018 from https://neurosciencenews.com/nitric-oxide-neurodegeneration-8753/[/cbtab][cbtab title=”Chicago”]University of Leicester “Discovery Sheds Light on How Neurodegenerative Diseases May Occur.” https://neurosciencenews.com/nitric-oxide-neurodegeneration-8753/ (accessed April 10, 2018).[/cbtab][/cbtabs]
Abstract
Nitric oxide-mediated posttranslational modifications control neurotransmitter release by modulating complexin farnesylation and enhancing its clamping ability
Nitric oxide (NO) regulates neuronal function and thus is critical for tuning neuronal communication. Mechanisms by which NO modulates protein function and interaction include posttranslational modifications (PTMs) such as S-nitrosylation. Importantly, cross signaling between S-nitrosylation and prenylation can have major regulatory potential. However, the exact protein targets and resulting changes in function remain elusive. Here, we interrogated the role of NO-dependent PTMs and farnesylation in synaptic transmission. We found that NO compromises synaptic function at the Drosophila neuromuscular junction (NMJ) in a cGMP-independent manner. NO suppressed release and reduced the size of available vesicle pools, which was reversed by glutathione (GSH) and occluded by genetic up-regulation of GSH-generating and de-nitrosylating glutamate-cysteine-ligase and S-nitroso-glutathione reductase activities. Enhanced nitrergic activity led to S-nitrosylation of the fusion-clamp protein complexin (cpx) and altered its membrane association and interactions with active zone (AZ) and soluble N-ethyl-maleimide-sensitive fusion protein Attachment Protein Receptor (SNARE) proteins. Furthermore, genetic and pharmacological suppression of farnesylation and a nitrosylation mimetic mutant of cpx induced identical physiological and localization phenotypes as caused by NO. Together, our data provide evidence for a novel physiological nitrergic molecular switch involving S-nitrosylation, which reversibly suppresses farnesylation and thereby enhances the net-clamping function of cpx. These data illustrate a new mechanistic signaling pathway by which regulation of farnesylation can fine-tune synaptic release.






