Finding has implications for alcoholism and other patterns of addictive behavior.
Research from the National Institutes of Health has identified neural circuits in mice that are involved in the ability to learn and alter behaviors. The findings help to explain the brain processes that govern choice and the ability to adapt behavior based on the end results.
Researchers think this might provide insight into patterns of compulsive behavior such as alcoholism and other addictions.
“Much remains to be understood about exactly how the brain strikes the balance between learning a behavioral response that is consistently rewarded, versus retaining the flexibility to switch to a new, better response,” said Kenneth R. Warren, Ph.D., acting director of the National Institute on Alcohol Abuse and Alcoholism. “These findings give new insight into the process and how it can go awry.”
The study, published online in Nature Neuroscience, indicates that specific circuits in the forebrain play a critical role in choice and adaptive learning.

Like other addictions, alcoholism is a disease in which voluntary control of behavior progressively diminishes and unwanted actions eventually become compulsive. It is thought that the normal brain processes involved in completing everyday activities become redirected toward finding and abusing alcohol.
The research, conducted by investigators from NIAAA, with support from the National Institute of Mental Health and the University of Cambridge, England, used a variety of approaches to study choice.
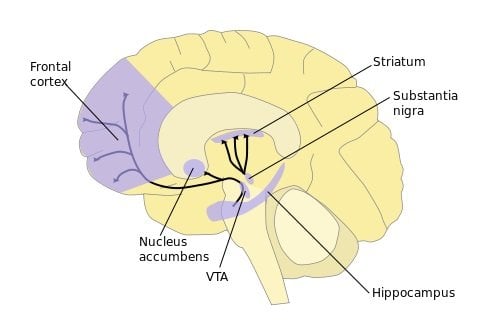
Researchers used a simple choice task in which mice viewed images on a computer touchscreen and learned to touch a specific image with their nose to get a food reward. Using various techniques to visualize and record neural activity, researchers found that as the mice learned to consistently make a choice, the brain’s dorsal striatum was activated. The dorsal striatum is thought to play an important role in motivation, decision-making, and reward.
Conversely, when the mice later had to shift to a new choice to receive a reward, the dorsal striatum quieted while regions in the prefrontal cortex, an area involved in decision-making and complex cognitive processes, became active.
Building upon these findings, the authors next deleted or pharmacologically blocked a component of nerve cells which normally binds the neurochemical glutamate (specifically, the GluN2B subunit of the NMDA receptor) within two different areas of the brain, the striatum and the frontal cortex. Previous studies have shown that GluN2B plays a role in memory, spatial reference, and attention. Researchers found that making dorsal striatal GluN2B inactive markedly slowed learning, while shutting down GluN2B in the prefrontal cortex made the mice less able to relearn the touchscreen reward task after the reward image was changed.
“These data add to what we understand about the neural control of behavioral flexibility and striatal learning by identifying GluN2B as a critical molecular substrate to both processes,” said the study’s senior author, Andrew Holmes, Ph.D., Laboratory Chief and Principal Investigator of the NIAAA Laboratory of Behavioral and Genomic Neuroscience.
“This is particularly intriguing for future studies because NMDA receptors are a major target for alcohol and contribute to important features of alcoholism, such as withdrawal. These new findings suggest that GluN2B in corticostriatal circuits may also play a key role in driving the transition from controlled drinking to compulsive abuse that characterizes alcoholism.”
Notes about this neuroscience and learning research
Contact: NIAAA Press Office – NIAAA/NIH
Source: NIAAA/NIH press release
Image Source: The dopamine pathway with the striatum and prefronal cortex highlighted diagram is credited to NIDA and is in the public domain.
Original Research: Abstract for “GluN2B in corticostriatal circuits governs choice learning and choice shifting” by Jonathan L Brigman, Rachel A Daut, Tara Wright, Ozge Gunduz-Cinar, Carolyn Graybeal, Margaret I Davis, Zhihong Jiang, Lisa M Saksida, Seiichiro Jinde, Matthew Pease, Timothy J Bussey, David M Lovinger, Kazu Nakazawa and Andrew Holmes in Nature Neuroscience. Published online July 7 2013 doi:10.1038/nn.3457