Summary: A new neuro-optics technique can manipulate memory consolidation in mouse models by hindering long-term potentiation. Researchers say eliminating local LTP in the hippocampus erased memories. The method could be used to isolate memory formation at the cellular level.
Source: Kyoto University
When an important document lands on your desk, you might file it away for safekeeping. The same thing happens with our memories: They first appear in one part of the brain and then move to another for long-term storage in a process known as memory consolidation.
Publishing in the journal Science, Kyoto University’s Akihiro Goto uses mouse brains to demonstrate a new neural-optic system to manipulate memories. The technique hinders nerve activity—known as long-term potentiation or LTP—which would otherwise consolidate memory during sleep.
LTP strengthens synapses through neural activity and is critical for memory formation. When and where memories are formed in the brain can be determined by examining when and which cells undergo LTP.
Drugs can disrupt LTP, but they have a general effect and are not good at targeting specific brain regions at specific time points in memory consolidation.
Looking for inspiration, Goto turned to Hollywood.
“In ‘Men in Black’ the agents erase memories with a light flash. We did something similar,” he says with a smile. His team uses light to deactivate proteins essential for LTP.
Switching the black suits and shades for white lab coats and safety goggles, co-author Yasunori Hayashi’s team illuminates mouse brains to inhibit cofilin, a protein essential for the synapse to function.
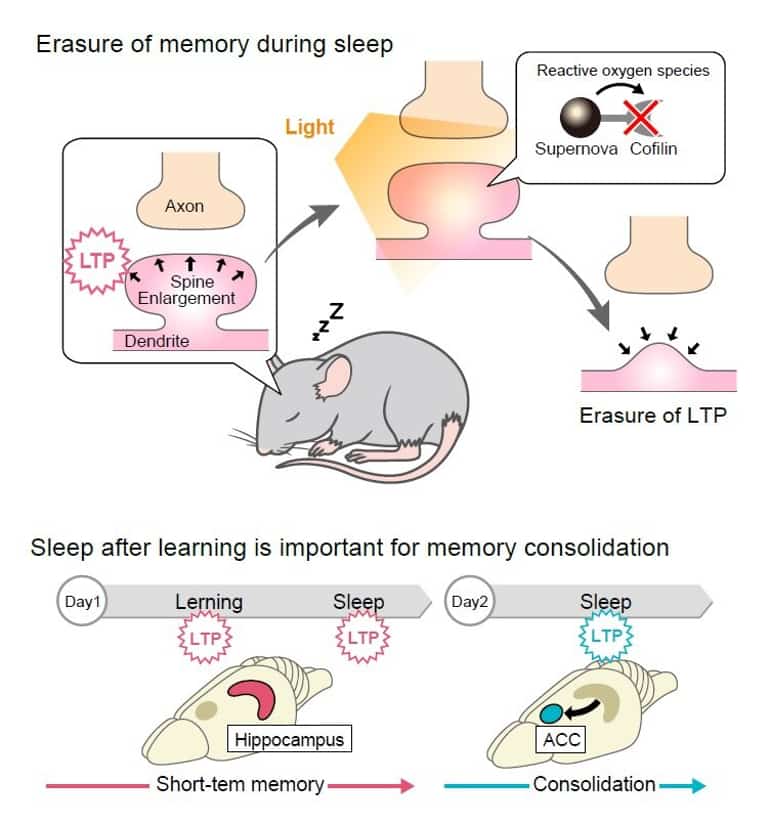
Initially, the brains are injected with the adeno-associated virus or AAV, commonly used for gene delivery, which then expresses a fused protein made from cofilin and fluorescent SuperNova. When exposed to light, these proteins release reactive oxygen that deactivates nearby compounds like cofilin.
The occurrence of LTP in the hippocampus, where memories are first stored, is significant. When this area of the brain is irradiated, once immediately after the mouse learns a task and then again during sleep after learning, the memory is lost.
“It was surprising that eliminating local LTP by targeted illumination clearly erased memory,” Goto comments.
Hayashi believes that this new technology provides a method for isolating memory formation both temporally and spatially in the brain at the cellular level.
Synaptic abnormalities related to LTP are involved in memory and learning disorders like Alzheimer’s disease and also psychiatric diseases like schizophrenia. Hayashi concludes, “We expect our method will lead to a range of treatments for mental disorders.”
About this neurotech and memory research news
Author: Press Office
Source: Kyoto University
Contact: Press Office – Kyoto University
Image: The image is credited to KyotoU / Akihiro Goto
Original Research: Closed access.
“Stepwise synaptic plasticity events drive the early phase of memory consolidation” by Akihiro Goto et al. Science
Abstract
Stepwise synaptic plasticity events drive the early phase of memory consolidation
Memories are initially encoded in the hippocampus but subsequently consolidated to the cortex. Although synaptic plasticity is key to these processes, its precise spatiotemporal profile remains poorly understood.
Using optogenetics to selectively erase long-term potentiation (LTP) within a defined temporal window, we found that distinct phases of synaptic plasticity play differential roles. The first wave acts locally in the hippocampus to confer context specificity.
The second wave, during sleep on the same day, organizes these neurons into synchronously firing assemblies. Finally, LTP in the anterior cingulate cortex during sleep on the second day is required for further stabilization of the memory.
This demonstrates the precise localization, timing, and characteristic contributions of the plasticity events that underlie the early phase of memory consolidation.