Summary: Ion channels use a “ball-and-chain” mechanism to help regulate ion flow. Findings confirm a long-standing hypothesis about ion channels and provide key advances in understanding the basic biological processes at work.
Source: Weill Cornell Medicine
Ion channels, which allow potassium and sodium ions to flow in and out of cells, are crucial in neuronal ‘firing’ in the central nervous system and for brain and heart function. These channels use a “ball-and-chain” mechanism to help regulate their ion flow, according to a new study led by Weill Cornell Medicine scientists.
The study, published March 18, 2020, in Nature, confirms a long-standing hypothesis about ion channels, and represents a key advance in the understanding of the basic biological processes at work in most cells.
The direct imaging of the ball-and-chain mechanism, using electron-microscopy techniques, can also provide a new angle to design drugs that target it to improve ion channel function. Ion channel abnormalities have been linked to a long list of disorders including epilepsies, heart arrhythmias, schizophrenia and diabetes.
“Scientists have been trying to get an atomic-scale picture of this mechanism since the 1970s, and now that we have it at last, it can become an important drug target,” said senior author Dr. Crina Nimigean, an associate professor of physiology and biophysics in anesthesiology at Weill Cornell Medicine.
Many types of ion channels, including those necessary for neuronal signaling and the beating of the heart, will physically open, allowing a flow of ions in or out of the cell, when a certain stimulus is applied. However, in order to switch ion flow on and off with high enough frequencies to meet the demands of neurons, heart muscle cells and other cell types, some ion channels need an additional, on-the-fly mechanism to stop ion flow–even when the stimulus is still present and the channel structure is in principle in the “open” state.
Researchers in the field have suspected since 1973, based on biochemical experiments, that this on-the-fly mechanism resembles a bathtub plug on a chain, or “ball-and-chain” structure. But confirming this directly with atomic-scale imaging methods has been a formidable challenge. This is due chiefly to the complexity of these channels in mammals and the difficulty of reconstructing them, for imaging purposes, in a cell-membrane-like environment where they are normally connected to other cell membrane components.
“Nobody knew exactly how this process actually looks and works–does the “ball” block the opening of the channel, or actually go in and plug the pore, or alternatively, alter the conformation of the channel indirectly?” said Dr. Nimigean.
She and her colleagues were able to overcome this challenge by imaging a potassium ion channel from Methanobacterium thermoautotrophicum, a bacteria-like species found at deep-sea geothermal vents. Its “MthK” channel is known to be structurally similar to the mammalian “BK” potassium channel that is crucial for the proper function of neurons and many other cell types–yet MthK has key simplifications that make it easier to image.
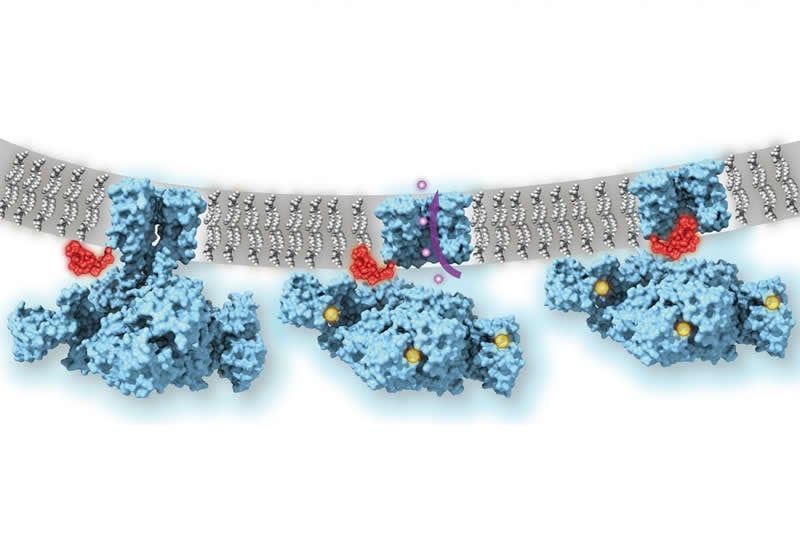
With low-temperature electron microscopy (cryo-EM), which bounces electrons instead of light off objects to make atomic-resolution images of them, the scientists obtained pictures of the MthK channel when it was switched open by calcium and switched closed. The pictures revealed that even when the MthK channel is in the calcium-activated, “open” state, the pathway through which ions flow was plugged by a flexible element that sticks into the pore of the channel structure.
The scientists confirmed the function of this plug mechanism by showing that when the ‘ball-and-chain’ was deleted genetically, the flow of potassium ions through the calcium-activated MthK channel was no longer regulated.
Dr. Nimigean and her colleagues now are planning to explore how this mechanism might be targeted therapeutically.
“Different classes of potassium channels in human cells are very similar in their channel structures. So a drug that blocks a particular channel will tend to affect other potassium channels and thus could have many unwanted side effects,” she said. “However, understanding and then targeting this ball-and-chain structure that we were able to image could allow us to therapeutically modulate potassium channels with much more specificity.”
Source:
Weill Cornell Medicine
Media Contacts:
Krystle Lopez – Weill Cornell Medicine
Image Source:
The image is credited to Dr. Crina Nimigean.
Original Research: Closed access
“Ball-and-chain inactivation in a calcium-gated potassium channel”. Chen Fan, Nattakan Sukomon, Emelie Flood, Jan Rheinberger, Toby W. Allen & Crina M. Nimigean .
Nature doi:10.1038/s41586-020-2116-0.
Abstract
Ball-and-chain inactivation in a calcium-gated potassium channel
Inactivation is the process by which ion channels terminate ion flux through their pores while the opening stimulus is still present1. In neurons, inactivation of both sodium and potassium channels is crucial for the generation of action potentials and regulation of firing frequency1,2. A cytoplasmic domain of either the channel or an accessory subunit is thought to plug the open pore to inactivate the channel via a ‘ball-and-chain’ mechanism3,4,5,6,7. Here we use cryo-electron microscopy to identify the molecular gating mechanism in calcium-activated potassium channels by obtaining structures of the MthK channel from Methanobacterium thermoautotrophicum—a purely calcium-gated and inactivating channel—in a lipid environment. In the absence of Ca2+, we obtained a single structure in a closed state, which was shown by atomistic simulations to be highly flexible in lipid bilayers at ambient temperature, with large rocking motions of the gating ring and bending of pore-lining helices. In Ca2+-bound conditions, we obtained several structures, including multiple open-inactivated conformations, further indication of a highly dynamic protein. These different channel conformations are distinguished by rocking of the gating rings with respect to the transmembrane region, indicating symmetry breakage across the channel. Furthermore, in all conformations displaying open channel pores, the N terminus of one subunit of the channel tetramer sticks into the pore and plugs it, with free energy simulations showing that this is a strong interaction. Deletion of this N terminus leads to functionally non-inactivating channels and structures of open states without a pore plug, indicating that this previously unresolved N-terminal peptide is responsible for a ball-and-chain inactivation mechanism.