Summary: Scientists have identified a previously unseen layered organization inside one of the brain’s most important memory hubs. The CA1 region of the hippocampus was found to contain four distinct bands of neuron types, each defined by unique genetic signatures that shift subtly across the structure.
This hidden architecture explains why different parts of CA1 support different behaviors such as memory, navigation, and emotion. The discovery also offers a new framework for understanding why specific neurons are more vulnerable in conditions like Alzheimer’s disease and epilepsy.
Key Facts:
- Four Distinct Neuron Layers: The CA1 region contains four continuous sheets of specialized neuron types rather than a gradual cellular blend.
- Single-Cell Gene Mapping: Researchers visualized over 330,000 RNA molecules across more than 58,000 neurons to build the atlas.
- Disease Relevance: The layered structure may explain selective neuron loss in Alzheimer’s, epilepsy, and other brain disorders.
Source: USC
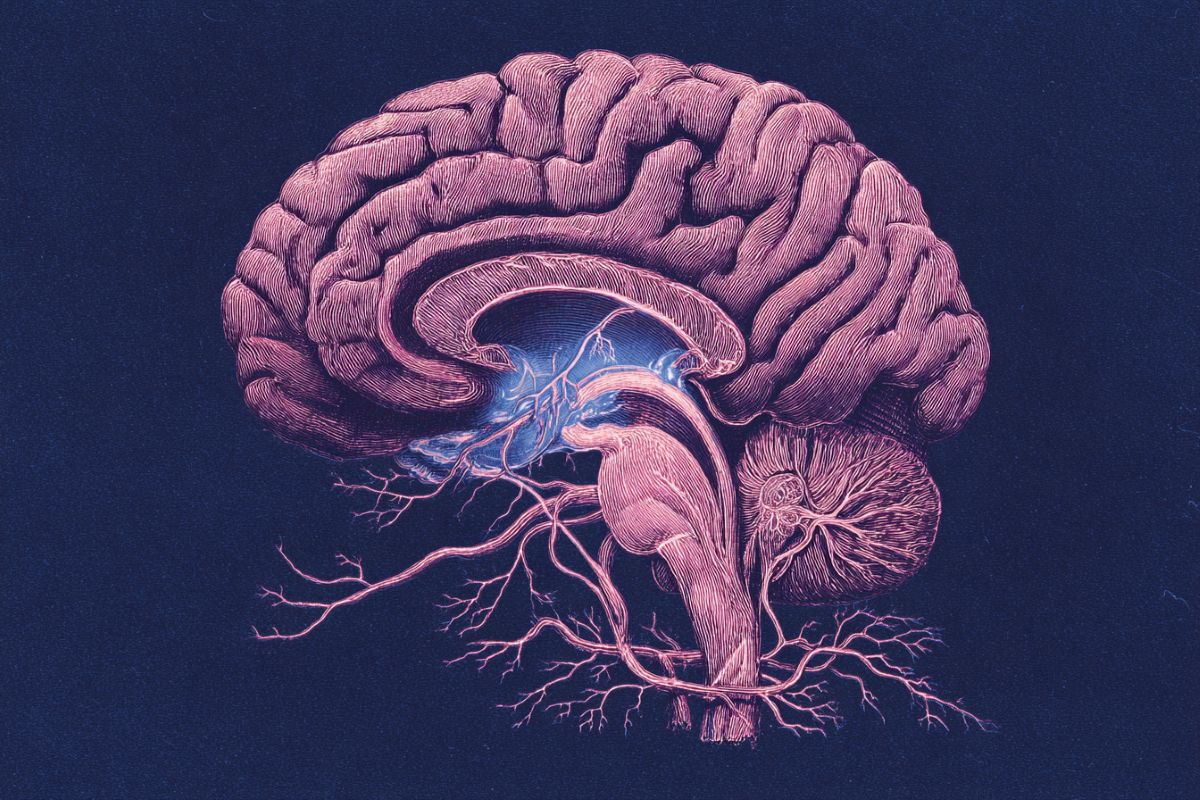
Researchers at the Mark and Mary Stevens Neuroimaging and Informatics Institute (Stevens INI) at the Keck School of Medicine of USC have identified a previously unknown pattern of organization in one of the brain’s most important areas for learning and memory.
The study, published in Nature Communications, reveals that the CA1 region of a mouse’s hippocampus, a structure vital for memory formation, spatial navigation, and emotions, has four distinct layers of specialized cell types.
This discovery changes our understanding of how information is processed in the brain and could explain why certain cells are more vulnerable in diseases like Alzheimer’s and epilepsy.
“Researchers have long suspected that different parts of the hippocampus’ CA1 region handle different aspects of learning and memory, but it wasn’t clear how the underlying cells were arranged,” said Michael S. Bienkowski, PhD, senior author of the study and assistant professor of physiology and neuroscience and of biomedical engineering.
“Our study shows that CA1 neurons are organized into four thin, continuous bands, each representing a different neuron type defined by a unique molecular signature. These layers aren’t fixed in place; instead, they subtly shift and change in thickness along the length of the hippocampus.
“This shifting pattern means that each part of CA1 contains its own mix of neuron types, which helps explain why different regions support different behaviors. This may also clarify why certain CA1 neurons are more vulnerable in conditions like Alzheimer’s disease and epilepsy: if a disease targets one layer’s cell type, the effects will vary depending on where in CA1 that layer is most prominent.”
Using a powerful RNA labeling method called RNAscope with high-resolution microscopy imaging, the team captured clear snapshots of single-molecule gene expression to identify CA1 cell types inside mouse brain tissue. Within 58.065 CA1 pyramidal cells, they visualized more than 330,000 RNA molecules—the genetic messages that show when and where genes are turned on.
By tracing these activity patterns, the researchers created a detailed map showing the borders between different types of nerve cells across the CA1 region of the hippocampus.
The results showed that the CA1 region consists of four continuous layers of nerve cells, each marked by a distinct set of active genes. In 3D, these layers form sheets that vary slightly in thickness and structure along the length of the hippocampus. This clear, layered pattern helps make sense of earlier studies that saw the region as a more gradual mix or mosaic of cell types.
“When we visualized gene RNA patterns at single-cell resolution, we could see clear stripes, like geological layers in rock, each representing a distinct neuron type,” said Maricarmen Pachicano, doctoral researcher at the Stevens INI’s Center for Integrative Connectomics and co–first author of the paper.
“It’s like lifting a veil on the brain’s internal architecture. These hidden layers may explain differences in how hippocampal circuits support learning and memory.”
The hippocampus is among the first regions affected in Alzheimer’s disease and is also implicated in epilepsy, depression, and other neurological conditions. By revealing the CA1’s layered structure, the study provides a roadmap to investigate which specific neuron types are most vulnerable in these disorders.
“Discoveries like this exemplify how modern imaging and data science can transform our view of brain anatomy,” said Arthur W. Toga, PhD, director of the Stevens INI and the Ghada Irani Chair in Neuroscience at the Keck School of Medicine of USC.
“This work builds on the Stevens INI’s long tradition of mapping the brain at every scale, from molecules to whole networks, and will inform both basic neuroscience and translational studies targeting memory and cognition.”
The new CA1 cell-type atlas, built using data from the Hippocampus Gene Expression Atlas (HGEA), is freely available to the global research community. The dataset includes interactive 3D visualizations accessible through the Schol-AR augmented-reality app, created at the Stevens INI, which allows scientists to explore hippocampal layers in unprecedented detail.
Because this layered pattern in mice resembles what has been seen in primate and human brains—including how the CA1 region changes in thickness—the researchers think it may be a common feature across many mammalian brains.
While additional studies are needed to confirm this organization in humans, the finding provides a promising foundation for future comparative and translational research into how hippocampal architecture supports memory and cognition.
“Understanding how these layers connect to behavior is the next frontier,” Bienkowski said. “We now have a framework to study how specific neuron layers contribute to such different functions like memory, navigation, and emotion, and how their disruption may lead to disease.”
About the study
In addition to Bienkowski and Pachicano, the study’s other authors include Shrey Mehta, Angela Hurtado, Tyler Ard, Jim Stanis, and Bayla Breningstall.
Funding: This work was supported by the National Institutes of Health/National Institute of Aging (K01AG066847, R36AG087310-01, supplement P30-AG066530-03S1), National Science Foundation (grant 2121164), and funding from the USC Center for Neuronal Longevity. Research data reported in this publication was supported by the Office of the Director, National Institutes of Health under award number S10OD032285.
Key Questions Answered:
A: Scientists discovered that the CA1 region is organized into four distinct layers of specialized neuron types rather than being a gradual mix of cells.
A: CA1 plays a central role in learning, memory formation, spatial navigation, and emotional processing.
A: Different layers may be selectively vulnerable in disorders like Alzheimer’s disease and epilepsy, helping explain why damage varies by region.
Editorial Notes:
- This article was edited by a Neuroscience News editor.
- Journal paper reviewed in full.
- Additional context added by our staff.
About this neuroscience research news
Author: Laura LeBlanc
Source: USC
Contact: Laura LeBlanc – USC
Image: The image is credited to Neuroscience News
Original Research: Open access.
“Laminar organization of pyramidal neuron cell types defines distinct CA1 hippocampal subregions” by Michael S. Bienkowski et al. Nature Communications
Abstract
Laminar organization of pyramidal neuron cell types defines distinct CA1 hippocampal subregions
Investigating the cell type organization of hippocampal CA1 is essential for understanding its role in memory and cognition and its susceptibility to neurological disorders like Alzheimer’s disease and epilepsy.
Multiple studies have identified different organizational principles for gene expression and how it reflects cell types within the CA1 pyramidal layer including gradients or mosaic.
Here, we identify sublaminar gene expression patterns within the mouse CA1 pyramidal layer that span across the entire hippocampal axis.
Our findings reveal that CA1 subregions (CA1d, CA1i, CA1v, CA1vv) contain differentially distributed layers of constituent cell types and can be identified by regional gene expression signatures.
This work offers a new perspective on the organization of CA1 cell types that can be used to further explore hippocampal cell types across species.