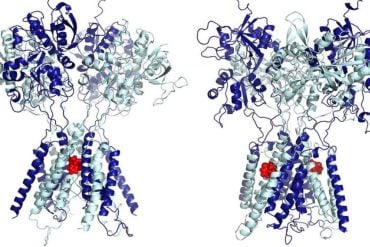
Summary: Researchers discover the DAG peptide can recognize specific protein elevation in brain blood vessels of both mouse models and humans with Alzheimer’s disease.
Source: SBP.
Researchers at Sanford Burnham Prebys Medical Discovery Institute (SBP) have identified a peptide that could lead to the early detection of Alzheimer’s disease (AD). The discovery, published in Nature Communications, may also provide a means of homing drugs to diseased areas of the brain to treat AD, Parkinson’s disease, as well as glioblastoma, brain injuries and stroke.
“Our goal was to find a new biomarker for AD,” says Aman Mann, Ph.D., research assistant professor at SBP who shares the lead authorship of the study with Pablo Scodeller, Ph.D., a postdoctoral researcher at SBP. “We have identified a peptide (DAG) that recognizes a protein that is elevated in the brain blood vessels of AD mice and human patients. The DAG target, connective tissue growth factor (CTGF) appears in the AD brain before amyloid plaques, the pathological hallmark of AD.”
“CTGF is a protein that is made in the brain in response to inflammation and tissue repair,” explains Mann. “Our finding that connects elevated levels of CTGF with AD is consistent with the growing body of evidence suggesting that inflammation plays an important role in the development of AD.”
The research team identified the DAG peptide using in vivo phage display screening at different stages of AD development in a mouse model. In young AD mice, DAG detected the earliest stage of the disease. If the early appearance of the DAG target holds true in humans, it would mean that DAG could be used as a tool to identify patients at early, pre-symptomatic stages of the disease when treatments already available may still be effective.
“Importantly, we showed that DAG binds to cells and brain from AD human patients in a CTGF-dependent manner” says Mann. “This is consistent with an earlier report of high CTGF expression in the brains of AD patients.”

“Our findings show that endothelial cells, the cells that form the inner lining of blood vessels, bind our DAG peptide in the parts of the mouse brain affected by the disease,” says Erkki Ruoslahti, M.D., Ph.D., distinguished professor at SBP and senior author of the paper. “This is very significant because the endothelial cells are readily accessible for probes injected into the blood stream, whereas other types of cells in the brain are behind a protective wall called the blood-brain barrier. The change in AD blood vessels gives us an opportunity to create a diagnostic method that can detect AD at the earliest stage possible.
“But first we need to develop an imaging platform for the technology, using MRI or PET scans to differentiate live AD mice from normal mice. Once that’s done successfully, we can focus on humans,” adds Ruoslahti.
“As our research progresses we also foresee CTGF as a potential therapeutic target that is unrelated to amyloid beta (Aβ), the toxic protein that creates brain plaques,” says Ruoslahti. “Given the number of failed clinical studies that have sought to treat AD patients by targeting Aβ, it’s clear that treatments will need to be given earlier–before amyloid plaques appear–or have to target entirely different pathways.
“DAG has the potential to fill both roles — identifying at risk individuals prior to overt signs of AD and targeted delivery of drugs to diseased areas of the brain. Perhaps CTGF itself can be a drug target in AD and other brain disorders linked to inflammation. We’ll just have to learn more about its role in these diseases”.
This technology has been licensed to a startup company, AivoCode Inc.
Funding: Funding sources for this research came, in part, from the Defense Advanced Research Projects Agency (DARPA), the European Research Council, the Wellcome Trust International Fellowship (WT095077MA), the European Regional Development Fund, the National Institutes of Health (P01 HD29587, R01 NS086890, DPI DA041722, and P30 NS076411), and a Distinguished Investigator Award of the Brain & Behavior Research Foundation.
Source: SBP
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Ruoslahti Lab, SBP.
Video Source: Video credited to SBP.
Original Research: Full open access research for “Identification of a peptide recognizing cerebrovascular changes in mouse models of Alzheimer’s disease” by Aman P. Mann, Pablo Scodeller, Sazid Hussain, Gary B. Braun, Tarmo Mölder, Kadri Toome, Rajesh Ambasudhan, Tambet Teesalu, Stuart A. Lipton & Erkki Ruoslahti in Nature Communications. Published online November 10 2017 doi:10.1038/s41467-017-01096-0
[cbtabs][cbtab title=”MLA”]SBP “Biomarker May Predict Early Alzheimer’s.” NeuroscienceNews. NeuroscienceNews, 10 November 2017.
<https://neurosciencenews.com/dag-alzheimers-biomarker-7914/>.[/cbtab][cbtab title=”APA”]SBP (2017, November 10). Biomarker May Predict Early Alzheimer’s. NeuroscienceNews. Retrieved November 10, 2017 from https://neurosciencenews.com/dag-alzheimers-biomarker-7914/[/cbtab][cbtab title=”Chicago”]SBP “Biomarker May Predict Early Alzheimer’s.” https://neurosciencenews.com/dag-alzheimers-biomarker-7914/ (accessed November 10, 2017).[/cbtab][/cbtabs]
Abstract
Identification of a peptide recognizing cerebrovascular changes in mouse models of Alzheimer’s disease
Cerebrovascular changes occur in Alzheimer’s disease (AD). Using in vivo phage display, we searched for molecular markers of the neurovascular unit, including endothelial cells and astrocytes, in mouse models of AD. We identified a cyclic peptide, CDAGRKQKC (DAG), that accumulates in the hippocampus of hAPP-J20 mice at different ages. Intravenously injected DAG peptide homes to neurovascular unit endothelial cells and to reactive astrocytes in mouse models of AD. We identified connective tissue growth factor (CTGF), a matricellular protein that is highly expressed in the brain of individuals with AD and in mouse models, as the target of the DAG peptide. We also showed that exogenously delivered DAG homes to the brain in mouse models of glioblastoma, traumatic brain injury, and Parkinson’s disease. DAG may potentially be used as a tool to enhance delivery of therapeutics and imaging agents to sites of vascular changes and astrogliosis in diseases associated with neuroinflammation.
“Identification of a peptide recognizing cerebrovascular changes in mouse models of Alzheimer’s disease” by Aman P. Mann, Pablo Scodeller, Sazid Hussain, Gary B. Braun, Tarmo Mölder, Kadri Toome, Rajesh Ambasudhan, Tambet Teesalu, Stuart A. Lipton & Erkki Ruoslahti in Nature Communications. Published online November 10 2017 doi:10.1038/s41467-017-01096-0