Summary: Six proteins in the blood can be used to assess a person’s risk of developing cerebral small vessel disease (CSVD). CSVD has been linked to an increased risk of stroke and Alzheimer’s disease.
Source: UCLA
A UCLA-led study has found that levels of six proteins in the blood can be used to gauge a person’s risk for cerebral small vessel disease, or CSVD, a brain disease that affects an estimated 11 million older adults in the U.S. CSVD can lead to dementia and stroke, but currently it can only be diagnosed with an MRI scan of the brain.
“The hope is that this will spawn a novel diagnostic test that clinicians can start to use as a quantitative measure of brain health in people who are at risk of developing cerebral small vessel disease,” said Dr. Jason Hinman, a UCLA assistant professor of neurology and lead author of the paper, which is published in the journal PLOS ONE.
CSVD is characterized by changes to the brain’s white matter — the areas of the brain that have a high concentration of myelin, a fatty tissue that insulates and protects the long extensions of brain cells. In CSVD, small blood vessels that snake through the white matter become damaged over time and the myelin begins to break down. This slows the communication between cells in the brain and can lead to problems with cognition and difficulty walking. And if the blood vessels become completely blocked, it can cause stroke.
The disease is also associated with a heightened risk for multiple forms of dementia, including Alzheimer’s disease.
Typically, doctors diagnose CSVD with an MRI scan after a person has experienced dementia or suffered a stroke. About a quarter of all strokes in the U.S. are associated with CSVD. But many cases of the disease go undiagnosed because of mild symptoms, such as trouble with walking or memory, that can often be attributed to normal aging.
In the new study, Hinman and colleagues focused on six proteins related to the immune system’s inflammatory response and centered on a molecule called interleukin-18, or IL-18. They hypothesized that inflammatory proteins that damage the brain in CSVD may be detectable in the bloodstream.
The researchers measured the levels of the proteins in the blood of 167 people whose average age was 76.4, and who had either normal cognition or mild cognitive impairment. As part of their voluntary participation in the study, 110 participants also underwent an MRI brain scan and 49 received a more advanced scan called diffusion tensor imaging.
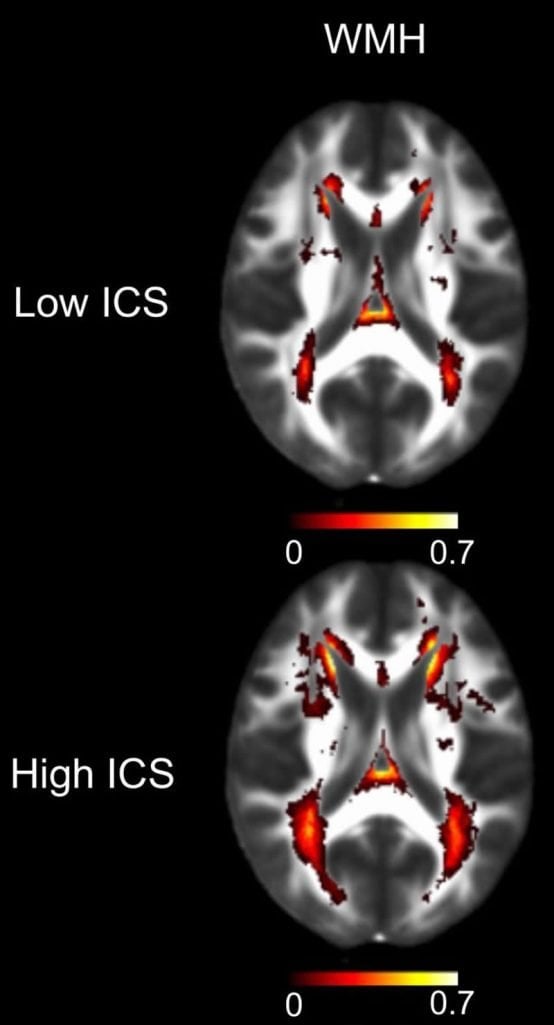
People whose MRI or diffusion tensor imaging tests showed signs of CSVD had significantly higher levels of the six blood proteins, the researchers discovered. If a person had higher-than-average levels of the six inflammatory proteins, they were twice as likely to have signs of CSVD on an MRI scan and 10% more likely to very early signs of white matter damage. Moreover, for every CSVD risk factor that a person had — such as high blood pressure, diabetes, or a previous stroke — the inflammatory protein levels in their blood were twice as high, on average.
To confirm the results, the team performed the blood test in a group with a much higher risk for CSVD: 131 people who visited a UCLA Health emergency department with signs of stroke. Once again, the blood test results were correlated with white matter changes in the brain that were detected by an MRI.
“I was pleasantly surprised that we were able to associate blood stream inflammation with CSVD in two fairly different populations,” Hinman said.
In MRI reports, the changes in the brain’s white matter caused by CSVD are usually only categorized in general terms — as mild, moderate or severe. The blood test is a step forward, Hinman said, because it provides a more quantitative scale for evaluating the disease. That means the blood test can be used to follow the progression of the disease or to identify people who are candidates for prevention efforts or treatments for CSVD.
“We’re hopeful that this will set the field on more quantitative efforts for CSVD so we can better guide therapies and new interventions,” Hinman said.
The blood test is not commercially available at this time.
The study’s first author is Marie Altendahl, a UCLA medical student who was at UC San Francisco at the time of the study. Other UCLA-affiliated authors are former undergraduate researcher Visesha Kakarla and former postdoctoral research fellow Guanxi Xiao. Other authors are from UC San Francisco and UC Davis.
Funding: The study was funded by the National Institutes of Health, the American Heart Association, the MarkVCID consortium and the Lillian R. Gleitsman Foundation.
Source:
UCLA
Media Contacts:
Marrecca Fiore – UCLA
Image Source:
The image is credited to UCLA Health.
Original Research: Open access
“An IL-18-centered inflammatory network as a biomarker for cerebral white matter injury”. Marie Altendahl, Pauline Maillard, Danielle Harvey, Devyn Cotter, Samantha Walters, Amy Wolf, Baljeet Singh, Visesha Kakarla, Ida Azizkhanian, Sunil A. Sheth, Guanxi Xiao, Emily Fox, Michelle You, Mei Leng, David Elashoff, Joel H. Kramer, Charlie Decarli, Fanny Elahi, Jason D. Hinman.
PLOS ONE doi:10.1371/journal.pone.0227835.
Abstract
An IL-18-centered inflammatory network as a biomarker for cerebral white matter injury
Chronic systemic sterile inflammation is implicated in the pathogenesis of cerebrovascular disease and white matter injury. Non-invasive blood markers for risk stratification and dissection of inflammatory molecular substrates in vivo are lacking. We sought to identify whether an interconnected network of inflammatory biomarkers centered on IL-18 and all previously associated with white matter lesions could detect overt and antecedent white matter changes in two populations at risk for cerebral small vessel disease. In a cohort of 167 older adults (mean age: 76, SD 7.1, 83 females) that completed a cognitive battery, physical examination, and blood draw in parallel with MR imaging including DTI, we measured cerebral white matter hyperintensities (WMH) and free water (FW). Concurrently, serum levels of a biologic network of inflammation molecules including MPO, GDF-15, RAGE, ST2, IL-18, and MCP-1 were measured. The ability of a log-transformed population mean-adjusted inflammatory composite score (ICS) to associate with MR variables was demonstrated in an age and total intracranial volume adjusted model. In this cohort, ICS was significantly associated with WMH (β = 0.222, p = 0.013), FW (β = 0.3, p = 0.01), and with the number of vascular risk factor diagnoses (r = 0.36, p<0.001). In a second cohort of 131 subjects presenting for the evaluation of acute neurologic deficits concerning for stroke, we used serum levels of 11 inflammatory biomarkers in an unbiased principal component analysis which identified a single factor significantly associated with WMH. This single factor was strongly correlated with the six component ICS identified in the first cohort and was associated with WMH in a generalized linear regression model adjusted for age and gender (p = 0.027) but not acute stroke. A network of inflammatory molecules driven by IL-18 is associated with overt and antecedent white matter injury resulting from cerebrovascular disease and may be a promising peripheral biomarker for vascular white matter injury.