Summary: Newly discovered modifications of the coronavirus RNA offers clues for combatting COVID-19. The findings also shed light on the lifecycle of the SARS-CoV-2 virus.
Source: Institute for Basic Science
Jean and Peter Medawar wrote in 1977 that a virus is “simply a piece of bad news wrapped up in proteins.” The “bad news” in the SARS-CoV-2 case is the new coronavirus carries its mysterious genome in the form of a very long ribonucleic acid (RNA) molecule. Grappling with COVID-19 pandemic, the world seems to be lost with no sense of direction in uncovering what this coronavirus (SARS-Cov-2) is composed of. Being an RNA virus, SARS-Cov-2 enters host cells and replicates a genomic RNA and produce many smaller RNAs (called “subgenomic RNAs”). These subgenomic RNAs are used for the synthesis of various proteins (spikes, envelopes, etc.) that are required for the beginning of SARS-Cov-2 lineage. Thus, the smaller RNAs make good targets for messing up new coronavirus’s conquering of our immune system. Though recent studies reported the sequence of the RNA genome, they only predicted where their genes might be, leaving the world still drown in disorientation.
Led by Professors KIM V. Narry and CHANG Hyeshik, the research team of the Center for RNA Research within the Institute for Basic Science (IBS), South Korea, succeeded in dissecting the architecture of SARS-CoV-2 RNA genome, in collaboration with Korea National Institute of Health (KNIH) within Korea Centers for Disease Control & Prevention (KCDC). The researchers experimentally confirmed the predicted subgenomic RNAs that are in turn translated into viral proteins. Furthermore, they analyzed the sequence information of each RNA and revealed where genes are exactly located on a genomic RNA. “Not only to detailing the structure of SARS-CoV-2, we also discovered numerous new RNAs and multiple unknown chemical modification on the viral RNAs. Our work provides a high-resolution map of SARS-CoV-2. This map will help understand how the virus replicates and how it escapes the human defense system,” explains Professor KIM V. Narry, the corresponding author of the study.
It was previously known that 10 subgenomic RNAs make up the viral particle structure. However, the research team confirmed that 9 subgenomic RNAs actually exist, invalidating the remaining one subgenomic RNA. Researchers also found that there are dozens of unknown subgenomic RNAs, owing to RNA fusion and deletion events. “Though it requires further investigation, these molecular events may lead to the relatively rapid evolution of coronavirus. Moreover, we find multiple unknown chemical modifications on the viral RNAs. It is unclear yet what these modifications do, but a possibility is that they may assist the virus to avoid the attack from the host,” says Prof. Kim.
The research team suggests that modified RNAs may have new properties that are different from unmodified RNAs even though they have the same genetic information in terms of RNA base sequence. They believe if they figure out the unknown characteristics of RNA, the findings may offer a new clue for combatting the new coronavirus. Newly discovered chemical modification will also help to understand the life cycle of the virus.
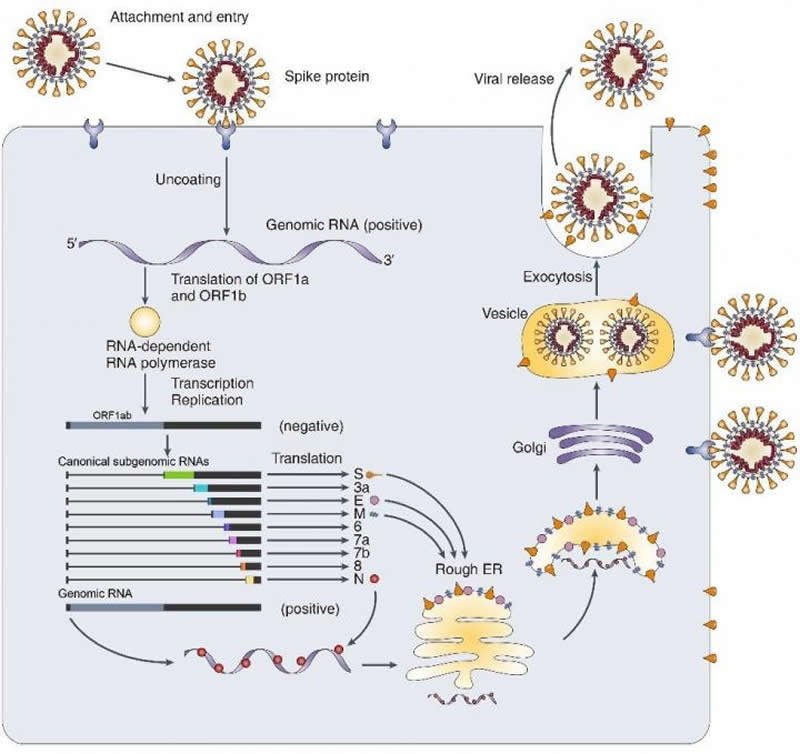
Behind the success of the study is the research team’s pairing of two complementary sequencing techniques; DNA nanoball sequencing and nanopore direct RNA sequencing. The nanopore direct RNA sequencing allows to directly analyze the entire long viral RNA without fragmentation. Conventional RNA sequencing methods usually require a step-by-step process of cutting and converting RNA to DNA before reading RNA. Meanwhile, the DNA nanoball sequencing can read only short fragments, but has the advantage of analyzing a large number of sequences with high accuracy. These two techniques turned out to be highly complementary to each other to analyze the viral RNAs.
“Now we have secured a high resolution gene map of the new coronavirus that guides us where to find each bit of genes on all of the total SARS-CoV-2 RNAs (transcriptome) and all modifications RNAs (epitranscriptome). It is time to explore the functions of the newly discovered genes and the mechanism underlying viral gene fusion. We also have to work on the RNA modifications to see if they play a role in virus replication and immune response. We firmly believe that our study will contribute to the development of diagnostics and therapeutics to combat the virus more effectively,” notes Professor KIM V. Narry.
About this coronavirus research article
Source:
Institute for Basic Science
Media Contacts:
V. Narry Kim – Institute for Basic Science
Image Source:
The image is credited to Institute for Basic Science.
Original Research: The study will appear in Cell.
Feel Free To Share This COVID-19 News.






