Manipulating molecule in the brain improves stress response, new target for depression treatment.
Increasing the levels of a signaling molecule found in the brain can positively alter response to stress, revealing a potential new therapeutic target for treatment of depression, UT Southwestern Medical Center researchers said.
The study, which appears in Nature Neuroscience, determined that elevating levels of the molecule cyclic adenosine monophosphate (cAMP) in brain cells had a positive impact on stress-induced behaviors in mice. Other studies have shown that patients with major depressive disorder often have impaired cAMP signaling and that chronic antidepressant treatments often turn on this signaling system.
“This is the first step in the development of a treatment for patients with major depressive disorder using this new strategy,” said senior author Dr. James Bibb, Professor of Psychiatry, and Neurology and Neurotherapeutics at UT Southwestern.
Major depressive disorder (MDD) may be triggered or exacerbated by severe or chronic stress. Depression affects more than 120 million people worldwide. Between 20 percent to 40 percent of people with depression are not helped by existing therapies, highlighting the need for new treatments and approaches.
The study was supported by the Center for Depression Research and Clinical Care at UT Southwestern. The Center, established with a $5 million lead gift from the Hersh Foundation earlier this year, combines basic research, translational clinical research in genetics, functional brain imaging, and treatment research across the entire age span, with a special focus on treatment of resistant, chronic, or recurrent depression.
“These exciting findings could help us develop very novel treatments to reduce stress response and prevent or treat depression effectively in the future,” said Dr. Madhukar Trivedi, Director of the Center for Depression Research and Clinical Care, Chief of the Division of Mood Disorders, Professor of Psychiatry, and holder of the Betty Jo Hay Distinguished Chair in Mental Health.
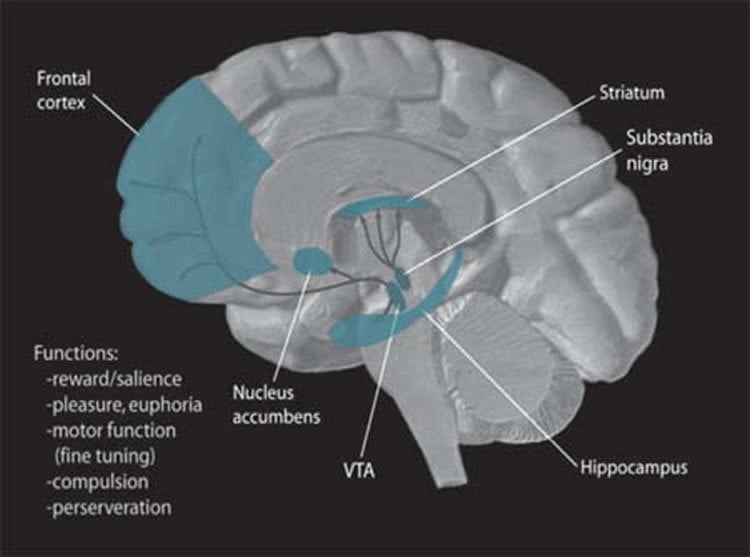
The chemical changes occurred in a region of the brain called the nucleus accumbens, which has a significant role in the processing of motivation, pleasure, and reward.
Researchers found that levels of cAMP can be elevated by disrupting the activation of an enzyme called phosphodiesterase-4 (PDE4). Knocking out the regulatory protein kinase Cdk5 in brain cells disrupted PDE4 function and elevated cAMP levels. This positively affected behavioral responses of the mice to stress-inducing experiments.
The researchers then developed a drug-like peptide that selectively blocked PDE4 function and increased the struggling response of mice to a test of acute stress commonly used to assess antidepressant efficacy.
Other UT Southwestern researchers who contributed to this study were lead author Dr. Florian Plattner, Instructor of Psychiatry; Dr. Ron Taussig, Associate Professor of Pharmacology; Adan Hernandez, senior research associate in Psychiatry; and Chungfeng Tan, research associate in Psychiatry.
Funding: This work was supported by the National Institutes of Health, the Brain and Behavior Research Foundation, National Research Service Award from the National Institute on Drug Abuse, and the California Metabolic Research Foundation.
Source: Russell Rian – UT Southwestern Medical Center
Image Credit: The image is credited to NIDA
Original Research: Abstract for “The role of ventral striatal cAMP signaling in stress-induced behaviors” by Florian Plattner, Kanehiro Hayashi, Adan Hernández, David R Benavides, Tara C Tassin, Chunfeng Tan, Jonathan Day, Maggy W Fina, Eunice Y Yuen, Zhen Yan, Matthew S Goldberg, Angus C Nairn, Paul Greengard, Eric J Nestler, Ronald Taussig, Akinori Nishi, Miles D Houslay and James A Bibb in Nature Neuroscience. Published online July 21 2015 doi:10.1038/nn.4066
Abstract
The role of ventral striatal cAMP signaling in stress-induced behaviors
The cAMP and cAMP-dependent protein kinase A (PKA) signaling cascade is a ubiquitous pathway acting downstream of multiple neuromodulators. We found that the phosphorylation of phosphodiesterase-4 (PDE4) by cyclin-dependent protein kinase 5 (Cdk5) facilitated cAMP degradation and homeostasis of cAMP/PKA signaling. In mice, loss of Cdk5 throughout the forebrain elevated cAMP levels and increased PKA activity in striatal neurons, and altered behavioral responses to acute or chronic stressors. Ventral striatum– or D1 dopamine receptor–specific conditional knockout of Cdk5, or ventral striatum infusion of a small interfering peptide that selectively targeted the regulation of PDE4 by Cdk5, produced analogous effects on stress-induced behavioral responses. Together, our results demonstrate that altering cAMP signaling in medium spiny neurons of the ventral striatum can effectively modulate stress-induced behavioral states. We propose that targeting the Cdk5 regulation of PDE4 could be a new therapeutic approach for clinical conditions associated with stress, such as depression.
“The role of ventral striatal cAMP signaling in stress-induced behaviors” by Florian Plattner, Kanehiro Hayashi, Adan Hernández, David R Benavides, Tara C Tassin, Chunfeng Tan, Jonathan Day, Maggy W Fina, Eunice Y Yuen, Zhen Yan, Matthew S Goldberg, Angus C Nairn, Paul Greengard, Eric J Nestler, Ronald Taussig, Akinori Nishi, Miles D Houslay and James A Bibb in Nature Neuroscience. Published online July 21 2015 doi:10.1038/nn.4066