Summary: Researchers report amyloid beta may change its internal structure to be absorbed into the cell and become toxic.
Source: WUSTL.
New research shows how amyloid beta enters brain cells.
Researchers have known that the peptide amyloid beta plays a role in causing Alzheimer’s disease, but they are still working to determine how it becomes toxic.
Jan Bieschke, a biomedical engineer at Washington University in St. Louis, and collaborators in Germany have found that amyloid beta must change its internal structure into a long, flat structure called a beta sheet to be absorbed into the cell and become toxic. Results of the research were published Sept. 9 in the Journal of Biological Chemistry.
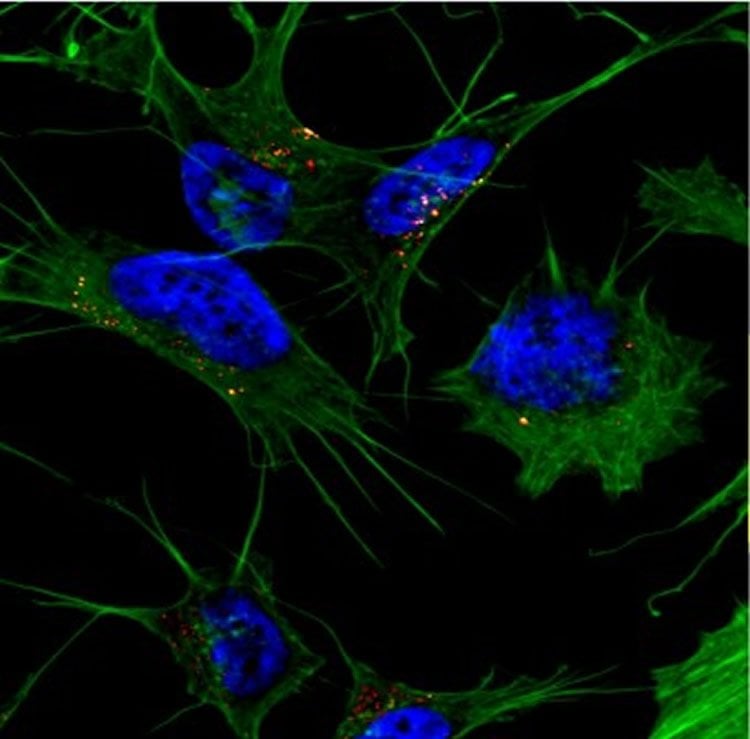
Bieschke, assistant professor of biomedical engineering in the School of Engineering & Applied Science, and his collaborators found that the amyloid beta protein structure that was able penetrate the cell had a specific type of beta sheet in which its peptides stacked onto each other, similar to a layer cake.
“Somewhere on this aggregation pathway, this type of structural element is formed for the amyloid beta to get into the cell,” Bieschke said. “There is a two-step process: amyloid beta can bind to the membrane and form aggregates while on the surface of the cell, then it gets taken up into the cell.”
Alzheimer’s researchers have had a long-standing debate on whether amyloid beta is toxic before entering the nerve cell or after entering the cell. Amyloid beta can interfere with the mitochondria, or the cell’s energy powerhouse. This causes the cell to stop breathing and leads to eventual cell death. Studies of patients with late-stage Alzheimer’s disease reveal the death of many nerve cells in the brain.
With this knowledge, Bieschke and his collaborators can investigate what happens next to amyloid beta once inside the cell and how it interacts with the mitochondria.
“We will determine if we can see and measure the interaction with the mitochondria membrane, and if these structures are interacting with mitochondria the same way as with the outer cell membrane,” he said. “Another question we will ask is: Can we manipulate the uptake or formation of these structures so they cannot enter the cell? This may be a therapeutic strategy to help future patients with Alzheimer’s.”
Source: Erika Ebsworth-Goold – WUSTL
Image Source: NeuroscienceNews.com image is credited to the researchers/WUSTL.
Original Research: Abstract for “Amyloid-β(1–42) Aggregation Initiates Its Cellular Uptake and Cytotoxicity” by Sha Jin, Niraja Kedia, Eva Illes-Toth, Ivan Haralampiev, Simon Prisner, Andreas Herrmann, Erich E. Wanker, and Jan Bieschke in Journal of Biological Chemistry. Published online September 9 2016 doi:10.1074/jbc.M115.691840
[cbtabs][cbtab title=”MLA”]WUSTL “Shape Shifting Protein Behind Alzheimer’s Disease.” NeuroscienceNews. NeuroscienceNews, 20 September 2016.
<https://neurosciencenews.com/alzheimers-protein-neurology-5083/>.[/cbtab][cbtab title=”APA”]WUSTL (2016, September 20). Shape Shifting Protein Behind Alzheimer’s Disease. NeuroscienceNew. Retrieved September 20, 2016 from https://neurosciencenews.com/alzheimers-protein-neurology-5083/[/cbtab][cbtab title=”Chicago”]WUSTL “Shape Shifting Protein Behind Alzheimer’s Disease.” https://neurosciencenews.com/alzheimers-protein-neurology-5083/ (accessed September 20, 2016).[/cbtab][/cbtabs]
Abstract
Amyloid-β(1–42) Aggregation Initiates Its Cellular Uptake and Cytotoxicity
The accumulation of amyloid β peptide(1–42) (Aβ(1–42)) in extracellular plaques is one of the pathological hallmarks of Alzheimer disease (AD). Several studies have suggested that cellular reuptake of Aβ(1–42) may be a crucial step in its cytotoxicity, but the uptake mechanism is not yet understood. Aβ may be present in an aggregated form prior to cellular uptake. Alternatively, monomeric peptide may enter the endocytic pathway and conditions in the endocytic compartments may induce the aggregation process. Our study aims to answer the question whether aggregate formation is a prerequisite or a consequence of Aβ endocytosis. We visualized aggregate formation of fluorescently labeled Aβ(1–42) and tracked its internalization by human neuroblastoma cells and neurons. β-Sheet-rich Aβ(1–42) aggregates entered the cells at low nanomolar concentration of Aβ(1–42). In contrast, monomer uptake faced a concentration threshold and occurred only at concentrations and time scales that allowed Aβ(1–42) aggregates to form. By uncoupling membrane binding from internalization, we found that Aβ(1–42) monomers bound rapidly to the plasma membrane and formed aggregates there. These structures were subsequently taken up and accumulated in endocytic vesicles. This process correlated with metabolic inhibition. Our data therefore imply that the formation of β-sheet-rich aggregates is a prerequisite for Aβ(1–42) uptake and cytotoxicity.
“Amyloid-β(1–42) Aggregation Initiates Its Cellular Uptake and Cytotoxicity” by Sha Jin, Niraja Kedia, Eva Illes-Toth, Ivan Haralampiev, Simon Prisner, Andreas Herrmann, Erich E. Wanker, and Jan Bieschke in Journal of Biological Chemistry. Published online September 9 2016 doi:10.1074/jbc.M115.691840






