Summary: Researchers have discovered an interaction in neurons that contributes to Parkinson’s disease.
Source: University of Alabama at Birmingham.
Researchers have found that an interaction between a mutant gene and alpha synuclein in neurons leads to hallmark pathologies seen in Parkinson’s disease, findings that may lead to new mechanisms and targets for neuroprotection.
Using a robust model for Parkinson’s disease, University of Alabama at Birmingham researchers and colleagues have discovered an interaction in neurons that contributes to Parkinson’s disease, and they have shown that drugs now under development may block the process.
The research team has shown that the most common genetic cause of Parkinson’s disease — a mutant LRRK2 kinase enzyme — contributes to the formation of inclusions in neurons, resembling one of the hallmark pathologies seen in Parkinson’s disease. These inclusions are made up of aggregated alpha synuclein protein, which — the research also shows — can be prevented from forming by using two LRRK2 kinase inhibitor drugs now being developed for clinical use.
The interaction between mutant LRRK2 kinase and alpha-synuclein “may uncover new mechanisms and targets for neuroprotection,” the researchers write in a recent Journal of Neuroscience paper. “These results demonstrate that alpha-synuclein inclusion formation in neurons can be blocked and that novel therapeutic compounds targeting this process by inhibiting LRRK2 kinase activity may slow progression of Parkinson’s disease-associated pathology.”
The potential clinical applications for novel neuroprotection strategies in LRRK2-linked Parkinson’s need to be tested in other preclinical models of Parkinson’s disease, say the researchers, led by corresponding author Laura A. Volpicelli-Daley, Ph.D., and senior author Andrew B. West, Ph.D., Center for Neurodegeneration and Experimental Therapeutics, UAB Department of Neurology.
“These data give us hope for the clinical potential of LRRK2 kinase inhibitors as effective therapies for Parkinson’s disease,” Volpicelli-Daley said. “The LRRK2 kinase inhibitors may inhibit the spread of pathologic alpha-synuclein, not only in patients with LRRK2 mutations, but in all Parkinson’s disease patients. Future studies to validate the safety and efficacy of the LRRK2 inhibitors will be necessary before testing the inhibitors in human clinical trials.”
Besides Parkinson’s disease, alpha-synuclein also plays a central role in development of dementia with Lewy bodies and multiple system atrophy, and it is associated with Alzheimer’s disease and other neurodegenerative disorders.
Research Details
The Parkinson’s disease model developed by Volpicelli-Daley applies very low concentrations of pre-formed fibrils of alpha-synuclein to in vitro or in vivo neurons. This causes formation of modified alpha-synuclein inclusions that share morphology with those found in the Parkinson’s disease brain after death.
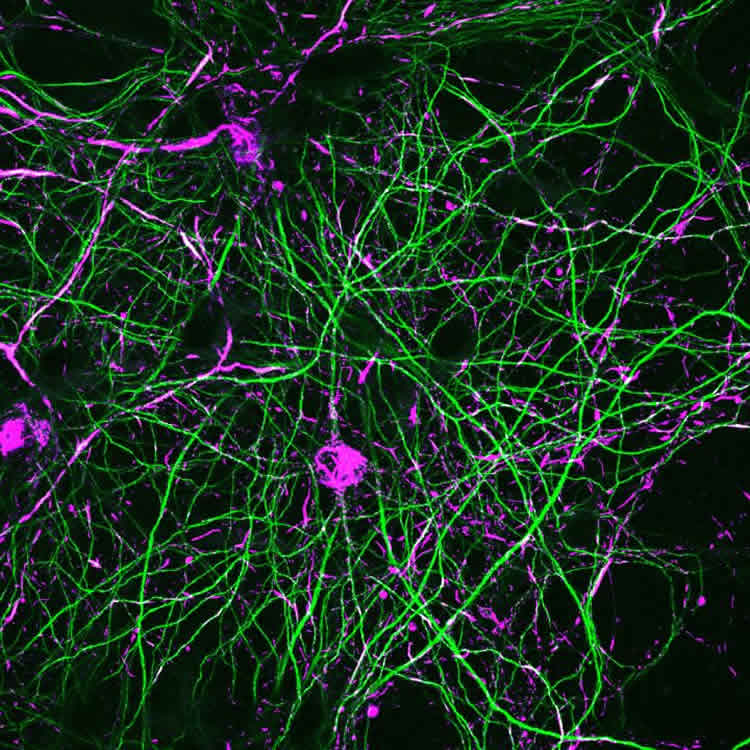
They used this model to test the effects of neuron expression of the mutant LRRK2 (“lark two”) kinase, G2019S-LRRK2, on the formation of the inclusion pathology.
They found that:
- G2019S-LRRK2 enhanced alpha-synuclein inclusions in primary hippocampal neurons from the hippocampus region of the brain, 18 days after fibril exposure, as compared with neurons that over-expressed normal LRRK2.
- The effects of G2019S-LRRK2 expression in the fibril-exposed neurons were lessened by very low concentrations of potent and selective preclinical drugs that inhibit LRRK2 kinase. This suggested that the kinase activity of G2019S-LRRK2, which adds a phosphate onto target proteins, underlies the faster formation of pathologic alpha-synuclein inclusions.
- G2019S-LRRK2 expression enhanced alpha-synuclein inclusion formation in dopamine neurons from the region of the brain called the substantia nigra pars compacta. The substantia nigra pars compacta is the area of the brain that dies in Parkinson’s disease, so this experiment further supports a link between the G2019S-LRRK2 mutation and Parkinson’s pathogenesis.
As a control, they used anti-sense oligonucleotides to knock down the expression endogenous alpha-synuclein in neurons that expressed G2019S-LRRK2, and this prevented formation of inclusions.
In fluorescence-recovery-after-photobleaching experiments, they found there was a larger pool of mobile alpha-synuclein, as opposed to membrane-bound alpha-synuclein, in neurons that expressed G2019S-LRRK2. Recent work by others has shown that mobile alpha-synuclein is prone to misfolding and aggregation, so the researchers hypothesize that the G2019S-LRRK2 mutation may contribute to Parkinson’s susceptibility by boosting the amounts of mobile alpha-synuclein in neurons.
Besides Volpicelli-Daley and West, co-authors of the paper “G2019S-LRRK2 expression augments alpha-synuclein sequestration into inclusions in neurons” are Hisham Abdelmotilib, Zhiyong Liu, Lindsay Stoyka, João Paulo Lima Daher and Kyle Fraser, all of the Center for Neurodegeneration and Experimental Therapeutics, UAB Department of Neurology; Austen J. Milnerwood, Centre for Applied Neurogenetics, University of British Columbia; Vivek K. Unni, Jungers Center for Neurosciences Research and Parkinson Center of Oregon, Oregon Health & Science University; Warren D. Hirst, Pfizer Neuroscience and Pain Research Unit, Cambridge, Massachusetts; Zhenyu Yue, Departments of Neurology and Neuroscience, Icahn School of Medicine at Mount Sinai; Hien T. Zhao, Ionis Pharmaceuticals, Carlsbad, California; and Richard E. Kennedy, Comprehensive Center for Healthy Aging and Division of Gerontology, Geriatrics, and Palliative Care, UAB Department of Medicine.
Volpicelli-Daley is an assistant professor in the Department of Neurology.
West is co-director of the Center for Neurodegeneration and Experimental Therapeutics, and the John A. and Ruth R. Jurenko Professor of Neurology at UAB.
Source: Jeff Hansen – University of Alabama at Birmingham
Image Source: This NeuroscienceNews.com image is credited to UAB.
Original Research: Abstract for “G2019S-LRRK2 Expression Augments α-Synuclein Sequestration into Inclusions in Neurons” by Laura A. Volpicelli-Daley, Hisham Abdelmotilib, Zhiyong Liu, Lindsay Stoyka, João Paulo Lima Daher, Austen J. Milnerwood, Vivek K. Unni, Warren D. Hirst, Zhenyu Yue, Hien T. Zhao, Kyle Fraser, Richard E. Kennedy, and Andrew B. West in Jounral of Neurosciece. Published online July 13 2016 doi:10.1523/JNEUROSCI.3642-15.2016
[cbtabs][cbtab title=”MLA”]University of Alabama at Birmingham. “Discovery May Lead to a Treatment to Slow Parkinson’s.” NeuroscienceNews. NeuroscienceNews, 19 July 2016.
<https://neurosciencenews.com/neurology-parkinsons-genetics-4706/>.[/cbtab][cbtab title=”APA”]University of Alabama at Birmingham. (2016, July 19). Discovery May Lead to a Treatment to Slow Parkinson’s. NeuroscienceNews. Retrieved July 19, 2016 from https://neurosciencenews.com/neurology-parkinsons-genetics-4706/[/cbtab][cbtab title=”Chicago”]University of Alabama at Birmingham. “Discovery May Lead to a Treatment to Slow Parkinson’s.” https://neurosciencenews.com/neurology-parkinsons-genetics-4706/ (accessed July 19, 2016).[/cbtab][/cbtabs]
Abstract
G2019S-LRRK2 Expression Augments α-Synuclein Sequestration into Inclusions in Neurons
Pathologic inclusions define α-synucleinopathies that include Parkinson’s disease (PD). The most common genetic cause of PD is the G2019S LRRK2 mutation that upregulates LRRK2 kinase activity. However, the interaction between α-synuclein, LRRK2, and the formation of α-synuclein inclusions remains unclear. Here, we show that G2019S-LRRK2 expression, in both cultured neurons and dopaminergic neurons in the rat substantia nigra pars compact, increases the recruitment of endogenous α-synuclein into inclusions in response to α-synuclein fibril exposure. This results from the expression of mutant G2019S-LRRK2, as overexpression of WT-LRRK2 not only does not increase formation of inclusions but reduces their abundance. In addition, treatment of primary mouse neurons with LRRK2 kinase inhibitors, PF-06447475 and MLi-2, blocks G2019S-LRRK2 effects, suggesting that the G2019S-LRRK2 potentiation of inclusion formation depends on its kinase activity. Overexpression of G2019S-LRRK2 slightly increases, whereas WT-LRRK2 decreases, total levels of α-synuclein. Knockdown of total α-synuclein with potent antisense oligonucleotides substantially reduces inclusion formation in G2019S-LRRK2-expressing neurons, suggesting that LRRK2 influences α-synuclein inclusion formation by altering α-synuclein levels. These findings support the hypothesis that G2019S-LRRK2 may increase the progression of pathological α-synuclein inclusions after the initial formation of α-synuclein pathology by increasing a pool of α-synuclein that is more susceptible to forming inclusions.
SIGNIFICANCE STATEMENT α-Synuclein inclusions are found in the brains of patients with many different neurodegenerative diseases. Point mutation, duplication, or triplication of the α-synuclein gene can all cause Parkinson’s disease (PD). The G2019S mutation in LRRK2 is the most common known genetic cause of PD. The interaction between G2019S-LRRK2 and α-synuclein may uncover new mechanisms and targets for neuroprotection. Here, we show that expression of G2019S-LRRK2 increases α-synuclein mobility and enhances aggregation of α-synuclein in primary cultured neurons and in dopaminergic neurons of the substantia nigra pars compacta, a susceptible brain region in PD. Potent LRRK2 kinase inhibitors, which are being developed for clinical use, block the increased α-synuclein aggregation in G2019S-LRRK2-expressing neurons. These results demonstrate that α-synuclein inclusion formation in neurons can be blocked and that novel therapeutic compounds targeting this process by inhibiting LRRK2 kinase activity may slow progression of PD-associated pathology.
“G2019S-LRRK2 Expression Augments α-Synuclein Sequestration into Inclusions in Neurons” by Laura A. Volpicelli-Daley, Hisham Abdelmotilib, Zhiyong Liu, Lindsay Stoyka, João Paulo Lima Daher, Austen J. Milnerwood, Vivek K. Unni, Warren D. Hirst, Zhenyu Yue, Hien T. Zhao, Kyle Fraser, Richard E. Kennedy, and Andrew B. West in Jounral of Neurosciece. Published online July 13 2016 doi:10.1523/JNEUROSCI.3642-15.2016