Scientists harness nature’s transport system to the brain.
Scientists from Duke-NUS Medical School (Duke-NUS) have derived a structural model of a transporter at the blood-brain barrier called Mfsd2a. This is the first molecular model of this critical transporter, and could prove important for the development of therapeutic agents that need to be delivered to the brain, across the blood-brain barrier. In future, this could help treat neurological disorders such as glioblastoma.
Currently, there are limitations to drug delivery to the brain as it is tightly protected by the blood-brain barrier. The blood-brain barrier is a protective barrier that separates the circulating blood from the central nervous system which can prevent the entry of certain toxins and drugs to the brain. This restricts the treatment of many brain diseases. However, as a transporter at the blood-brain barrier, Mfsd2a is a potential conduit for drug delivery directly to the brain, thus bypassing the barrier.
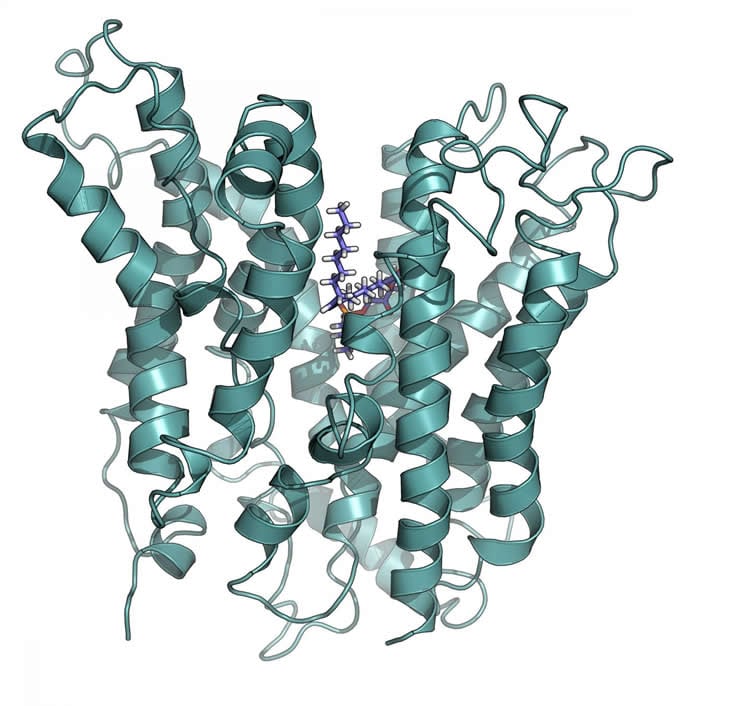
In this study, recently published in the Journal of Biological Chemistry, first author Duke-NUS MD/PhD student Debra Quek and senior author Professor David Silver used molecular modeling and biochemical analyses of altered Mfsd2a transporters to derive a structural model of human Mfsd2a. Importantly, the work identifies new binding features of the transporter, providing insight into the transport mechanism of Mfsd2a.
“Our study provides the first glimpse into what Mfsd2a looks like and how it might transport essential lipids across the blood-brain barrier,” said Ms Quek. “It also facilitates a structure-guided search and design of scaffolds for drug delivery to the brain via Mfsd2a, or of drugs that can be directly transported by Mfsd2a.”
Currently this information is being used by Duke-NUS researchers to design novel therapeutic agents for direct drug delivery across the blood brain barrier for the treatment of neurological diseases. This initiative by the Centre for Technology and Development (CTeD) at Duke-NUS, is one of many collaborative research efforts aimed at translating Duke-NUS’ research findings into tangible commercial and therapeutic applications for patients.
Ms Quek plans to further validate her findings by purifying the Mfsd2a protein in order to further dissect how it functions as a transporter.
Funding: Research supported by the ational Research Foundation Singapore, Singapore Ministry of Education.
Source: Dharshini Subbiah – Duke-NUS Medical School
Image Source: The image is credited to Duke-NUS Medical School.
Original Research: Abstract for “Structural insights into the transport mechanism of the human sodium-dependent lysophosphatidylcholine transporter Mfsd2a” by Debra Q.Y. Quek, Long N. Nguyen, Hao Fan and David L. Silver in Journal of Biological Chemistry. Published online March 4 2016 doi:10.1074/jbc.M116.721035
Abstract
Structural insights into the transport mechanism of the human sodium-dependent lysophosphatidylcholine transporter Mfsd2a
Major Facilitator Superfamily Domain containing 2A (Mfsd2a) was recently characterized as a sodium-dependent lysophosphatidylcholine (LPC) transporter expressed at the blood-brain barrier endothelium. It is the primary route for importation of docosohexaenoic acid and other long-chain fatty acids into foetal and adult brain, and is essential for mouse and human brain growth and function. Remarkably, Mfsd2a is the first identified MFS family member that uniquely transports lipids, implying that Mfsd2a harbours unique structural features and transport mechanism. Here, we present three 3D structural models of human Mfsd2a derived by homology modelling using MelB- and LacY-based crystal structures, and refined by biochemical analysis. All models revealed 12 transmembrane helices and connecting loops, and represented the partially outward-open, outward-partially occluded, and inward-open states of the transport cycle. In addition to a conserved sodium-binding site, three unique structural features were identified: A phosphate headgroup binding site, a hydrophobic cleft to accommodate a hydrophobic hydrocarbon tail, and three sets of ionic locks that stabilize the outward-open conformation. Ligand docking studies and biochemical assays identified Lys436 as a key residue for transport. It is seen forming a salt bridge with the negative charge on the phosphate headgroup. Importantly, Mfsd2a transported structurally related acylcarnitines but not a lysolipid without a negative charge, demonstrating the necessity of a negative charged headgroup interaction with Lys436 for transport. These findings support a novel transport mechanism by which LPCs are flipped within the transporter cavity by pivoting about Lys436 leading to net transport from the outer to the inner leaflet of the plasma membrane.
“Structural insights into the transport mechanism of the human sodium-dependent lysophosphatidylcholine transporter Mfsd2a” by Debra Q.Y. Quek, Long N. Nguyen, Hao Fan and David L. Silver in Journal of Biological Chemistry. Published online March 4 2016 doi:10.1074/jbc.M116.721035







