Summary: Researchers have developed a new, light sensitive technique that allows for visualization of neuromodulation in unprecedented detail.
Source: Max Planck Florida Institute for Neuroscience.
Researchers at Max Planck Florida Institute for Neuroscience developed a light-sensitive technique to visualize and manipulate neuromodulation with unprecedented spatial and temporal precision.
When we think of neuronal communication, we often picture a single neuron releasing molecular neurotransmitters into a junction called a synapse where they stimulate another neuron. But sometimes, instead of crossing a synapse, neurotransmitters flow widely throughout the brain – flooding different types of receptors and stimulating many other neurons at a time. In this scenario, they are known as neuromodulators. Identifying and manipulating specific neurons impacted by neuromodulation has been a challenge for researchers. Hyungbae Kwon’s lab at Max Planck Florida Institute for Neuroscience (MPFI), and collaborators from University of Geneva, Korea University and Max Planck Institute of Neurobiology, have recently developed a technique with a newly designed gene expression system that allows researchers to visualize and manipulate neuromodulation in real time.
The Inducible Tango technique, called iTango, builds upon the Tango system, a technique developed nearly a decade ago. With the Tango technique, individual neurons that had been stimulated by neuromodulators would express a green fluorescent protein, so researchers could identify them. However, once sensor proteins are overexpressed, it continuously produces fluorescence regardless of neuromodulators, meaning that over time as more and more neurons would fluoresce and researchers wouldn’t be able to tell which populations of neurons were stimulated. Because of this limitation, the Tango technique was not practical for studying neuromodulatory states in mammals.
iTango uses a light-sensitive labeling system. Cells will only fluoresce if the particular neuromodulator is present and the researchers are simultaneously shining a special blue light on the neurons of interest. Once the researchers turn the light off, the protein stops glowing. The Kwon Lab’s reengineering of the original Tango system with a “light switch” gives researchers control over the timing of the fluorescence so that the signal to noise ratio is increased over ten-fold, making it much easier for scientists to identify specific populations of neurons in model species such as mice. The work led by Drs. Dongmin Lee, Meaghan Creed and Kanghoon Jung was published in Nature Methods in April 2017.
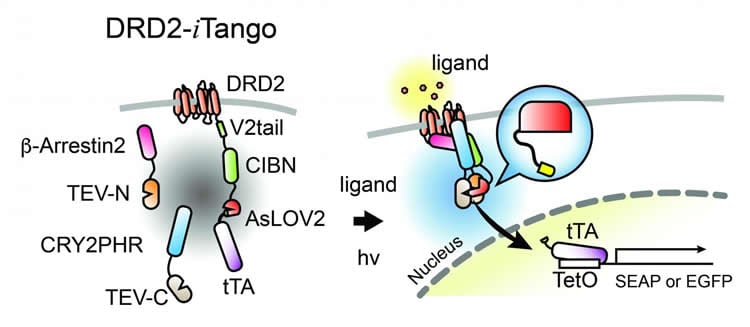
In this study, Hyungbae Kwon’s team used iTango to identify specific neurons in a mouse’s brain that were activated by neuromodulatory dopamine during specific animal behaviors. They were able to identify two populations of neurons – one involved in the increased locomotion and one involved in the reward feeling. His team took the research a step further – expressing ion channels sensitive to light, they were able to selectively inhibit or induce behaviors associated with the neuronal populations they had previously identified. Furthermore, they were able to control cocaine-responsive behavior by using the iTango technique.
Dr. Kwon explains that while his laboratory is primarily interested in basic neuroscience and the ways in which neuromodulators impact neuronal circuits and their formation, he anticipates that the iTango method will be applied to drug development, targeted cancer therapy, and many other research areas. One of the molecules that comprises the iTango system is a G-protein sensor that could be replaced by any G-protein. G-proteins are common targets for pharmacological drugs so companies could use this technique to screen potential drug candidates with incredible specificity. Sometimes viable drug candidates get thrown out because the signal-to-noise ratio in the existing screening method is very low. Because the light-sensitivity of the label increases the signal-to-noise ratio over ten times, the iTango technique has the potential to clearly identify these overlooked candidates without requiring any additional time investment.
“The most important part of this story is that we made this technique light sensitive. The light sensitivity provides a huge advantage because you can apply it to basically any signaling,” said Dr. Kwon. “iTango will be useful for visualizing and manipulating the neuronal circuitry that underlies drug-induced behaviors and psychiatric diseases related to neuromodulation, such as mood disorders or schizophrenia.”
Funding: Swiss National Science Foundation, Academic Society of Geneva, Private Foundation of the Geneva University Hospital, European Research Council, Kil Chung Hee Fellowship, Max Planck Florida Institute for Neuroscience funded this study.
Source: Jennifer Gutierrez – Max Planck Florida Institute for Neuroscience
Image Source: NeuroscienceNews.com image is credited to Max Planck Florida Institute for Neuroscience.
Video Source: The video is credited to Max Planck Florida Institute for Neuroscience.
Original Research: Abstract for “Temporally precise labeling and control of neuromodulatory circuits in the mammalian brain” by Dongmin Lee, Meaghan Creed, Kanghoon Jung, Thomas Stefanelli, Daniel J Wendler, Won Chan Oh, Neymi Layne Mignocchi, Christian Lüscher & Hyung-Bae Kwon in Nature Methods.10.1038/nmeth.423410.1016/j.mayocp.2017.02.014
[cbtabs][cbtab title=”MLA”]Max Planck Florida Institute for Neuroscience “New Technique Studies Neuromodulation in Real Time.” NeuroscienceNews. NeuroscienceNews, 3 April 2017.
<https://neurosciencenews.com/itango-neuromodulation-6330/>.[/cbtab][cbtab title=”APA”]Max Planck Florida Institute for Neuroscience (2017, April 3). New Technique Studies Neuromodulation in Real Time. NeuroscienceNew. Retrieved April 3, 2017 from https://neurosciencenews.com/itango-neuromodulation-6330/[/cbtab][cbtab title=”Chicago”]Max Planck Florida Institute for Neuroscience “New Technique Studies Neuromodulation in Real Time.” https://neurosciencenews.com/itango-neuromodulation-6330/ (accessed April 3, 2017).[/cbtab][/cbtabs]
Abstract
Temporally precise labeling and control of neuromodulatory circuits in the mammalian brain
Few tools exist to visualize and manipulate neurons that are targets of neuromodulators. We present iTango, a light- and ligand-gated gene expression system based on a light-inducible split tobacco etch virus protease. Cells expressing the iTango system exhibit increased expression of a marker gene in the presence of dopamine and blue-light exposure, both in vitro and in vivo. We demonstrated the iTango system in a behaviorally relevant context, by inducing expression of optogenetic tools in neurons under dopaminergic control during a behavior of interest. We thereby gained optogenetic control of these behaviorally relevant neurons. We applied the iTango system to decipher the roles of two classes of dopaminergic neurons in the mouse nucleus accumbens in a sensitized locomotor response to cocaine. Thus, the iTango platform allows for control of neuromodulatory circuits in a genetically and functionally defined manner with spatial and temporal precision.
“Temporally precise labeling and control of neuromodulatory circuits in the mammalian brain” by Dongmin Lee, Meaghan Creed, Kanghoon Jung, Thomas Stefanelli, Daniel J Wendler, Won Chan Oh, Neymi Layne Mignocchi, Christian Lüscher & Hyung-Bae Kwon in Nature Methods.10.1038/nmeth.423410.1016/j.mayocp.2017.02.014






