Nerve cells have to react extremely quickly, but depending on the task they are supposed to perform they often need to work more slowly. Berlin scientists have now shown that a receptor in the synapse can adapt to follow both regimes. It’s another example of the amazing flexibility of the brain, manifested at the molecular level.
Day after day, the brain performs incredible feats – whether it is predicting the path of a ball as we dive to catch it, processing sound waves or saving memories over decades. This range of performance is possible, because nerve cells react extremely rapidly and can produce up to 1000 electrical impulses per second, but their repertoire also includes slower reactions in which the impulses are infrequent. Many labs throughout the world are investigating how nerve cells can behave so flexibly. Two scientists at the Leibniz-Institut für Molekulare Pharmakologie in Berlin (FMP) have now made a surprising discovery: flexibility is found already at the molecular level. The most common receptor at our synapses can switch between two different types of behaviour, depending on the incoming signals. This is the AMPA-type glutamate receptor, which responds to glutamate released by neighbouring cells. This chemical handshake allows nerve impulses to “jump” from one cell to the next. For over 30 years, the AMPA receptor has been considered to be a true specialist for following rapid impulses: in less than a millisecond, it can switch back and forth between its closed and open forms. However, Anna Carbone and Andrew Plested, members of the NeuroCure Cluster of Excellence who work at the FMP, have now shown that repeated activation can push the receptor into a slow mode, accompanied by high activity. In this state of “superactivation”, it can stay open for up to a second.
The origin of this research was the chance mutation of a laboratory mouse, many years ago. The “Stargazer mouse” first attracted attention for its epileptic seizures, during which it would pull back its head and look up in the air as if in a trance. Later, it was discovered that the “Stargazer mouse” lacks a specific protein that normally forms a complex with the AMPA receptor. The protein was christened Stargazin, and, like many before them, the two FMP researchers experimented with it in cell cultures. They used the patch clamp technique to record the flow of current through receptors that formed complexes with Stargazin. They changed the properties of the receptor by mutating it, in order to see how the complex behaved. In the process, they found several unexpected forms of activity. Their explanatory model, which has now been published in Nature Communications, is simple. According to this model, the AMPA receptor is fundamentally in rapid mode, but can be switched to a slow mode by collateral activation of Stargazin. This “superactivation” is a kind of short-term memory on the molecular level, through which a positive feedback loop is created.
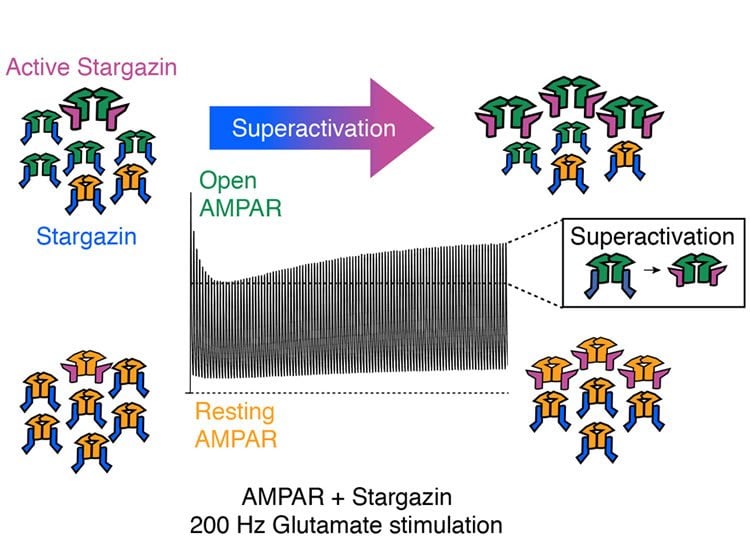
Positive feedback loops are vitally important in biology. For instance, one example is the Ferguson reflex, which helps to support contractions during childbirth. “As far as I know, it’s the first time anyone has shown that a positive feedback loop can develop within a single molecular complex. The advantage is that superactivation can build up and subside rapidly” says Andrew Plested. Normally, feedback loops only set in after minutes or hours. However, in the case of the AMPA receptor, the superactivation can start in less than a second. “The idea that the AMPA receptor might also work in slow mode has been circulating for some time, but until now nobody has managed to explain this phenomenon,” says Andrew Plested. The group is now working full steam ahead to demonstrate what role superactivation might play in the brain. Their model may help us to better understand synapses and the plasticity of the brain, and in the long term it may make a contribution to the treatment of neurological diseases.
Source: FMP
Image Credit: The image is credited to Andrew Plested
Original Research: Full open access research for “Superactivation of AMPA receptors by auxiliary proteins” by Anna L. Carbone and Andrew J. R. Plested in Nature Communications. Published online January 8 2016 doi:10.1038/ncomms10178
Abstract
Superactivation of AMPA receptors by auxiliary proteins
Glutamate receptors form complexes in the brain with auxiliary proteins, which control their activity during fast synaptic transmission through a seemingly bewildering array of effects. Here we devise a way to isolate the activation of complexes using polyamines, which enables us to show that transmembrane AMPA receptor regulatory proteins (TARPs) exert their effects principally on the channel opening reaction. A thermodynamic argument suggests that because TARPs promote channel opening, receptor activation promotes AMPAR-TARP complexes into a superactive state with high open probability. A simple model based on this idea predicts all known effects of TARPs on AMPA receptor function. This model also predicts unexpected phenomena including massive potentiation in the absence of desensitization and supramaximal recovery that we subsequently detected in electrophysiological recordings. This transient positive feedback mechanism has implications for information processing in the brain, because it should allow activity-dependent facilitation of excitatory synaptic transmission through a postsynaptic mechanism.
“Superactivation of AMPA receptors by auxiliary proteins” by Anna L. Carbone and Andrew J. R. Plested in Nature Communications. Published online January 8 2016 doi:10.1038/ncomms10178






