Summary: Sleep deprivation increases the number of available A1 adenosine receptors, but restorative sleep helps normalize them again, a new study reports.
Source: Forschungszentrum Jülich.
In a new study, scientists from Forschungszentrum Jülich together with partners from the German Aerospace Center (DLR) have investigated the molecular changes with which the human brain reacts to exceptionally long wake phases. The test subjects stayed awake for 52 hours and then had their brains scanned at Jülich’s PET Centre. Subsequently, they were taken to DLR in Cologne, where – monitored by the scientists – they were able to catch up on their sleep for 14 hours.
Lack of sleep can severely affect our performance and health. Moreover, a lack of sleep causes changes in the brain which the researchers were able to measure in their experiment. “Our investigations have shown that sleep deprivation increases the number of available A1 adenosine receptors. Thanks to the subsequent sleep phase, they then normalized back to the initial level,” reports PD Dr. David Elmenhorst from Jülich’s Institute of Neuroscience and Medicine (INM-2).
The A1 adenosine receptors are built into the cell wall as a type of receiver. Their function is to forward the signal from adenosine, the docking chemical messenger, to the interior of the cell, where it decreases the cell’s activity. It is thought that not only the adenosine itself but also the A1 receptors are responsible for the urge to sleep, which becomes stronger the longer a person stays awake. Adenosine is an elementary product of the energy metabolism. Its concentration varies practically second by second. The number of free receptors, in contrast, changes much more slowly and thus seems better suited for a kind of “sleep memory”.
Resistant to sleep deprivation
The effect of caffeine is also associated with this type of receptor. The stimulant accumulates at complex protein molecules and blocks them. In this series of experiments, the test subjects had to do without coffee and other invigorating substances. During their 52-hour wake phase, they were subjected to several performance tests: pressing buttons to measure their reaction time and memorizing words to determine their memory performance. One striking feature was the individual differences in performance: some of the sleep-deprived participants displayed extreme lapses, sometimes lasting several seconds, while in others a performance drop was hardly measurable. Such a predisposition could be advantageous for jobs in which people regularly have to perform reliably in spite of lacking sleep.
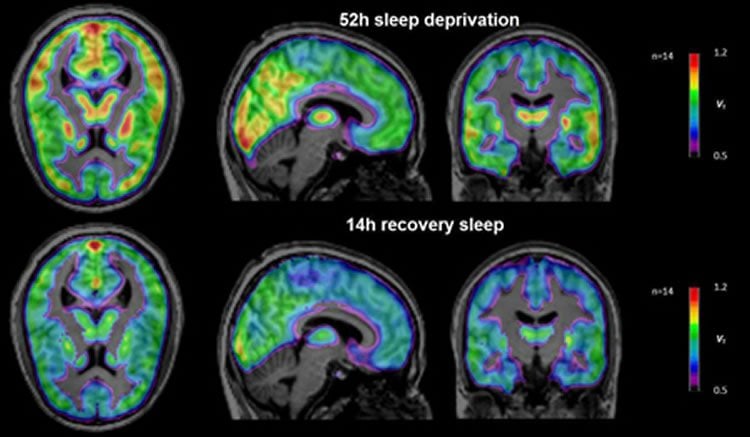
“Astonishingly, we did not measure a constant value of A1 receptor density in this seemingly resistant group of test subjects, but a large increase,” reports David Elmenhorst. The higher value does not correspond to an exceptionally high concentration of receptor molecules, however, since positron emission tomography (PET) records only a net value. Tracer molecules in the blood stream of the test subjects dock to free receptor molecules and can be observed in the PET scanner when they decay. In this manner, only those receptors are recorded that are not blocked and therefore available at the time of measurement. “Our theory is, therefore, that the test subjects with high A1 receptor density produce relatively little adenosine and thus inhibit the cell activity to a lesser degree,” says Elmenhorst. Consequently, the total number of free receptors is higher at the time of the PET measurement.
Relevant for treating depression
These findings are also of relevance for clinical medicine: sleep deprivation is a quick tool against depression, but only effective for a short time. “There are many efforts to increase the duration of the therapeutic effects of sleep deprivation in the treatment of depression. But the problem so far is that when people sleep again just once they often fall back into their depressed state,” says David Elmenhorst. A better understanding of the interrelations between mood and adenosine regulation could thus contribute to optimizing the design of wake therapies.
Source: Forschungszentrum Jülich
Image Source: NeuroscienceNews.com images are credited to Forschungszentrum Jülich / Ralf-Uwe Limbach.
Original Research: Abstract for “Recovery sleep after extended wakefulness restores elevated A1 adenosine receptor availability in the human brain” by David Elmenhorst, Eva-Maria Elmenhorst, Eva Hennecke, Tina Kroll, Andreas Matusch, Daniel Aeschbach, and Andreas Bauer PNAS. Published online April 3 2017 doi:10.1073/pnas.1614677114
[cbtabs][cbtab title=”MLA”]Forschungszentrum Jülich “How the Brain Reacts to Sleep Deprivation.” NeuroscienceNews. NeuroscienceNews, 5 April 2017.
<https://neurosciencenews.com/sleep-deprivation-neuroimaging-6343/>.[/cbtab][cbtab title=”APA”]Forschungszentrum Jülich (2017, April 5). How the Brain Reacts to Sleep Deprivation. NeuroscienceNew. Retrieved April 5, 2017 from https://neurosciencenews.com/sleep-deprivation-neuroimaging-6343/[/cbtab][cbtab title=”Chicago”]Forschungszentrum Jülich “How the Brain Reacts to Sleep Deprivation.” https://neurosciencenews.com/sleep-deprivation-neuroimaging-6343/ (accessed April 5, 2017).[/cbtab][/cbtabs]
Abstract
Recovery sleep after extended wakefulness restores elevated A1 adenosine receptor availability in the human brain
Adenosine and functional A1 adenosine receptor (A1AR) availability are supposed to mediate sleep–wake regulation and cognitive performance. We hypothesized that cerebral A1AR availability after an extended wake period decreases to a well-rested state after recovery sleep. [18F]CPFPX positron emission tomography was used to quantify A1AR availability in 15 healthy male adults after 52 h of sleep deprivation and following 14 h of recovery sleep. Data were additionally compared with A1AR values after 8 h of baseline sleep from an earlier dataset. Polysomnography, cognitive performance, and sleepiness were monitored. Recovery from sleep deprivation was associated with a decrease in A1AR availability in several brain regions, ranging from 11% (insula) to 14% (striatum). A1AR availabilities after recovery did not differ from baseline sleep in the control group. The degree of performance impairment, sleepiness, and homeostatic sleep-pressure response to sleep deprivation correlated negatively with the decrease in A1AR availability. Sleep deprivation resulted in a higher A1AR availability in the human brain. The increase that was observed after 52 h of wakefulness was restored to control levels during a 14-h recovery sleep episode. Individuals with a large increase in A1AR availability were more resilient to sleep-loss effects than those with a subtle increase. This pattern implies that differences in endogenous adenosine and A1AR availability might be causal for individual responses to sleep loss.
“Recovery sleep after extended wakefulness restores elevated A1 adenosine receptor availability in the human brain” by David Elmenhorst, Eva-Maria Elmenhorst, Eva Hennecke, Tina Kroll, Andreas Matusch, Daniel Aeschbach, and Andreas Bauer PNAS. Published online April 3 2017 doi:10.1073/pnas.1614677114






