Summary: Study reveals how sleep deprivation can greatly affect soldiers on the battlefield.
Source: US Army Research Laboratory
New Army-funded study looks at effects of sleep deprivation, which can greatly affect Soldiers on the battlefield.
Research conducted at the University of Rochester Medical Center and funded by the Army Research Office, an element of the U.S. Army Combat Capabilities Development Command’s Army Research Laboratory, suggests that people who rely on sleeping during daytime hours are at greater risk for developing neurological disorders.
The study, published in Nature Communications, details how the complex set of molecular and fluid dynamics that comprise the glymphatic system – the brain’s unique process of waste removal – are synchronized with the master internal clock that regulates the sleep-wake cycle.
“Establishing a role for communication between astrocytes and the significant impacts of circadian timing on glymphatic clearance dynamics represent a major step in understanding the fundamental process of waste clearance regulation in the brain,” said Dr. Frederick Gregory, a program manager for ARO’s neurophysiology of cognition initiative. “This knowledge is crucial to developing future countermeasures that offset the deleterious effects of sleep deprivation and addresses future multi-domain military operation requirements for Soldiers to sustain performance over longer periods without the ability to rest.”
The glymphatic system, first discovered by the URMC Nedergaard lab in 2012, consists of a network that piggybacks on the brain’s blood circulation system and is comprised of layers of plumbing, with the inner blood vessel encased by a ‘tube’ that transports cerebrospinal fluid. The system pumps the fluid through brain tissue primarily during sleep, washing away toxic proteins and other waste.
“These findings show that glymphatic system function is not solely based on sleep or wakefulness, but by the daily rhythms dictated by our biological clock,” said neuroscientist Maiken Nedergaard, M.D., D.M.Sc., co-director of the Center for Translational Neuromedicine at URMC and senior author of the study.
The research team and others have shown the role that blood pressure, heart rate, circadian timing, and depth of sleep play in the glymphatic system’s function and the chemical signaling that occurs in the brain to turn the system on and off. They have also shown how disrupted sleep or trauma can cause the system to break down and allow toxic proteins to accumulate in the brain, potentially giving rise to a number of neurodegenerative diseases, such as Alzheimer’s.
Circadian rhythms, 24-hour body clocks, are maintained in a small area of the brain called the suprachiasmatic nucleus. This clock regulates several important biological functions, including the sleep-wake cycle.
The new study, conducted in mice, showed that when the animals were anesthetized all day long, their glymphatic system still only functioned during their typical rest period – mice are nocturnal, so their sleep-wake cycle is the opposite of humans.
“Circadian rhythms in humans are tuned to a day-wake, night-sleep cycle,” said Dr. Lauren Hablitz, first author of the new study and a research assistant professor in the Center for Translational Neuromedicine at URMC. “Because this timing also influences the glymphatic system, these findings suggest that people who rely on cat naps during the day to catch up on sleep or work the night shift may be at risk for developing neurological disorders. In fact, clinical research shows that individuals who rely on sleeping during daytime hours are at much greater risk for Alzheimer’s and dementia along with other health problems.”
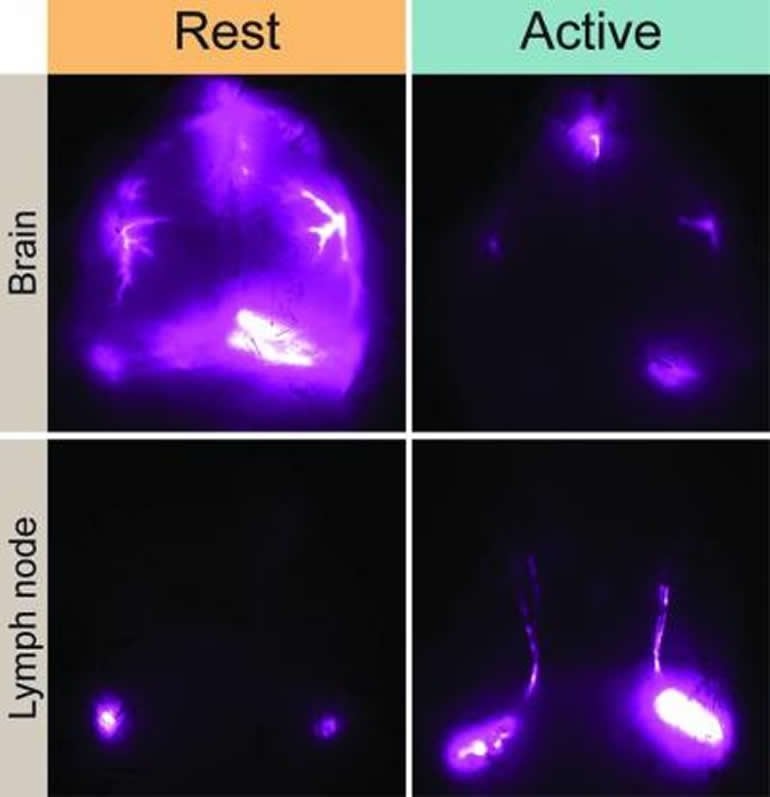
The study singles out cells called astrocytes that play multiple functions in the brain. Scientists believe that astrocytes in the suprachiasmatic nucleus help regulate circadian rhythms. Astrocytes also serve as gatekeepers that control the flow of cerebrospinal fluid throughout the central nervous system. The results of the study suggest that communication between astrocytes in different parts of the brain may share the common goal of optimizing the glymphatic system’s function during sleep.
The researchers also found that during wakefulness, the glymphatic system diverts cerebrospinal fluid to lymph nodes in the neck. Because the lymph nodes are key waystations in the regulation of the immune system, the research suggests that cerebrospinal fluid may represent a fluid clock that helps wake up the body’s infection fighting capabilities during the day.
Funding: In addition to the Army, the National Institute of Neurological Disorders and Stroke, the National Institute of Aging, and the Novo Nordisk and Lundbeck Foundations supported the research.
About this sleep research article
Source:
US Army Research Laboratory
Contacts:
Lisa B Bistreich-Wolfe – US Army Research Laboratory
Image Source:
The image is credited to University of Rochester Medical Center.
Original Research: Open access
“Circadian control of brain glymphatic and lymphatic fluid flow” by Lauren M. Hablitz, Virginia Plá, Michael Giannetto, Hanna S. Vinitsky, Frederik Filip Stæger, Tanner Metcalfe, Rebecca Nguyen, Abdellatif Benrais & Maiken Nedergaard. Nature Communications.
Abstract
Circadian control of brain glymphatic and lymphatic fluid flow
The glymphatic system is a network of perivascular spaces that promotes movement of cerebrospinal fluid (CSF) into the brain and clearance of metabolic waste. This fluid transport system is supported by the water channel aquaporin-4 (AQP4) localized to vascular endfeet of astrocytes. The glymphatic system is more effective during sleep, but whether sleep timing promotes glymphatic function remains unknown. We here show glymphatic influx and clearance exhibit endogenous, circadian rhythms peaking during the mid-rest phase of mice. Drainage of CSF from the cisterna magna to the lymph nodes exhibits daily variation opposite to glymphatic influx, suggesting distribution of CSF throughout the animal depends on time-of-day. The perivascular polarization of AQP4 is highest during the rest phase and loss of AQP4 eliminates the day-night difference in both glymphatic influx and drainage to the lymph nodes. We conclude that CSF distribution is under circadian control and that AQP4 supports this rhythm.






