Summary: Researchers observe molecules moving along the length of neural stem cells.
Source: Duke University.
Scientists visualize potential mechanisms driving formation of new neurons in the brain.
Duke University scientists have caught the first glimpse of molecules shuttling along a sort of highway running the length of neural stem cells, which are crucial to the development of new neurons.
This new view has given them an intriguing clue that a protein deficient in Fragile X syndrome, an autism-related disorder that causes intellectual disability, is responsible for moving at least some of this molecular cargo up and down the stem cells. The findings appear online Dec. 1 in Current Biology.
“The moving molecules we saw in these stem cells could be crucial for their function — including their decision to become neurons,” said the study’s senior investigator Debra Silver, an assistant professor of molecular genetics and microbiology at the Duke University School of Medicine. “We’re excited about these new discoveries and have many more questions.”
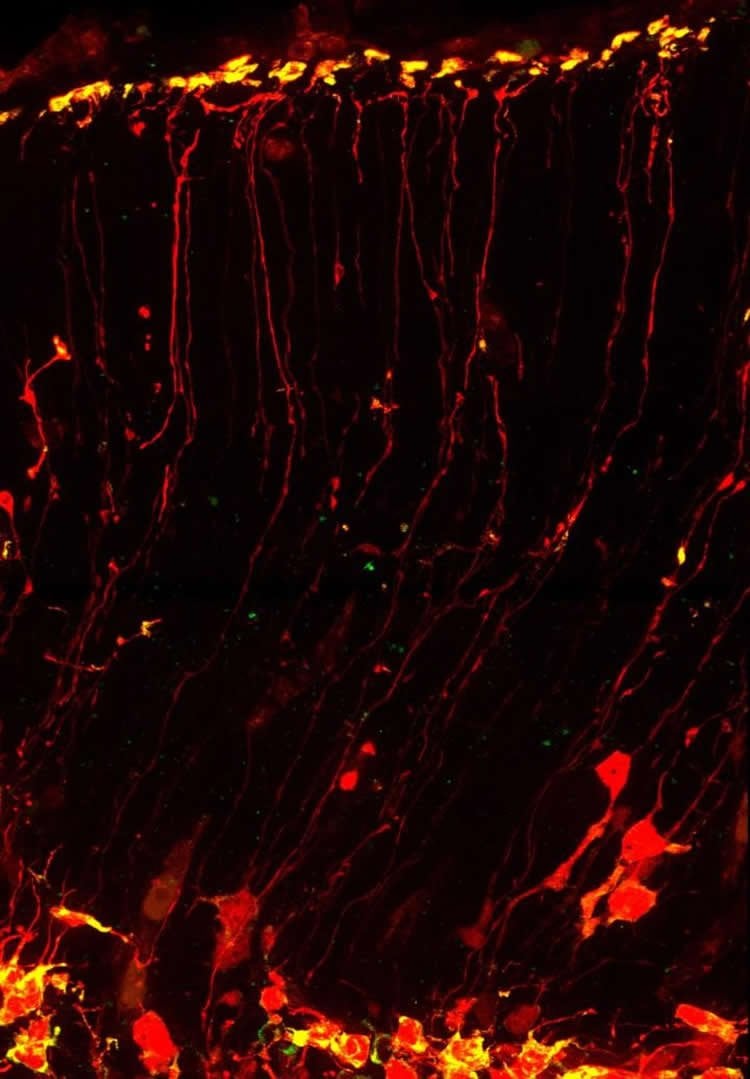
Neural stem cells are buried deep within the brain and project long, thin extensions outward. The ends of these cells, called “endfeet,” go so far as to reach the uppermost layer of the brain and prevent neurons, which climb up these thin strands, from migrating further.
Compared to the cell’s main body, these far-flung endfeet are living in a vastly different environment of the brain. Their surroundings may influence whether a neural stem cell generates another stem cell or becomes a neuron. That determination affects how many neurons the brain can generate.
These cells are so long that researchers thought that, like neurons, they would need to ship some of their contents long distances, including the messenger RNA molecule that is needed to manufacture proteins.
After toiling with the microscope, postdoctoral researcher Louis-Jan Pilaz was, for the first time, able to see mRNAs moving down the neural stem cell’s shaft, frame-by-frame.
“The [fluorescently tagged mRNAs] were stopping sometimes, and then they would keep going, like they have an intention,” Pilaz said. “No one had seen molecules moving at this speed within neural stem cells before.”
The results suggest that neural stem cells are highways for molecular transport, carrying not just mRNAs but also many other types of proteins, Silver said.
When mRNA reaches the endfeet it gets translated into protein by other cellular machinery. Silver’s group was able to show this definitively with a new test they developed that allowed them to isolate the endfeet from the rest of the cell. Using fluorescent tagging, graduate student Ashley Lennox was able to visualize new proteins being made within the endfeet.
“[Until now], there have been really limited tools for being able to evaluate this in an intact tissue, and that’s where our study provides a new model,” Silver said.
Silver’s team knew that mRNA was shipped in a controlled fashion to the endfeet, rather than merely diffusing, but they didn’t know which other molecules might control these steps. They examined a handful of molecules known to influence RNA dynamics and were surpised to find that the Fragile X syndrome protein called FMRP bound and carried the mRNAs.
Previous studies from several groups have implicated FMRP in neural stem cell function, but very little was understood about its role in brain development.
The group found 115 different mRNAs that FMRP latched onto: about 30 percent of these are linked to brain diseases, and about half of those are enriched in autism. They picked one of these mRNAs and showed, using a mouse model of Fragile X, that mRNA needed FMRP to arrive at the neural stem cell’s endfeet.
“This was really exciting. We clearly saw enrichment of a subset of RNAs, that we were able to go on and validate using other methods,” Silver said.
The group is conducting further studies to understand how production of protein is controlled within the endfeet, and whether it changes over the course of development. The scientists are also working to parse FMRP’s different functions to gauge their effects on brain development.
Funding: The research was supported by Fay/Frank Seed grant from the Brain Research Foundation.
Source: Karl Bates – Duke University
Image Source: NeuroscienceNews.com image is credited to Louis-Jan Pilaz, Duke University.
Original Research: Abstract for “Dynamic mRNA Transport and Local Translation in Radial Glial Progenitors of the Developing Brain” by Louis-Jan Pilaz, Ashley L. Lennox, Jeremy P. Rouanet, and Debra L. Silver in Current Biology. Published online October 19 2016 doi:/10.1016/j.cub.2016.10.040
[cbtabs][cbtab title=”MLA”]Duke University. “Neural Stem Cells Serve as RNA Highways Too.” NeuroscienceNews. NeuroscienceNews, 1 December 2016.
<https://neurosciencenews.com/rna-neural-stem-cells-5656/>.[/cbtab][cbtab title=”APA”]Duke University. (2016, December 1). Neural Stem Cells Serve as RNA Highways Too. NeuroscienceNews. Retrieved December 1, 2016 from https://neurosciencenews.com/rna-neural-stem-cells-5656/[/cbtab][cbtab title=”Chicago”]Duke University. “Neural Stem Cells Serve as RNA Highways Too.” https://neurosciencenews.com/rna-neural-stem-cells-5656/ (accessed December 1, 2016).[/cbtab][/cbtabs]
Abstract
Dynamic mRNA Transport and Local Translation in Radial Glial Progenitors of the Developing Brain
Highlights
•The radial glia basal process is a highway for active directed transport of RNA
•mRNA is locally translated in radial glia endfeet hundreds of micrometers from the soma
•Endfeet FMRP-bound RNAs encode autism-related signaling and cytoskeletal regulators
•FMRP controls RNA localization and active mRNA transport in radial glia
Summary
In the developing brain, neurons are produced from neural stem cells termed radial glia. Radial glial progenitors span the neuroepithelium, extending long basal processes to form endfeet hundreds of micrometers away from the soma. Basal structures influence neuronal migration, tissue integrity, and proliferation. Yet, despite the significance of these distal structures, their cell biology remains poorly characterized, impeding our understanding of how basal processes and endfeet influence neurogenesis. Here we use live imaging of embryonic brain tissue to visualize, for the first time, rapid mRNA transport in radial glia, revealing that the basal process is a highway for directed molecular transport. RNA- and mRNA-binding proteins, including the syndromic autism protein FMRP, move in basal processes at velocities consistent with microtubule-based transport, accumulating in endfeet. We develop an ex vivo tissue preparation to mechanically isolate radial glia endfeet from the soma, and we use photoconvertible proteins to demonstrate that mRNA is locally translated. Using RNA immunoprecipitation and microarray analyses of endfeet, we discover FMRP-bound transcripts, which encode signaling and cytoskeletal regulators, including many implicated in autism and neurogenesis. We show FMRP controls transport and localization of one target, Kif26a. These discoveries reveal a rich, regulated local transcriptome in radial glia, far from the soma, and establish a tractable mammalian model for studying mRNA transport and local translation in vivo. We conclude that cytoskeletal and signaling events at endfeet may be controlled through translation of specific mRNAs transported from the soma, exposing new mechanistic layers within stem cells of the developing brain.
“Dynamic mRNA Transport and Local Translation in Radial Glial Progenitors of the Developing Brain” by Louis-Jan Pilaz, Ashley L. Lennox, Jeremy P. Rouanet, and Debra L. Silver in Current Biology. Published online October 19 2016 doi:/10.1016/j.cub.2016.10.040






