Summary: Researchers report they have successfully transplanted retinal pigment cells derived from stem cells without rejection or the need of immunosuppressant drugs.
Source: RIKEN.
Researchers at the RIKEN Center for Developmental Biology (CDB) have successfully transplanted retinal pigment cells derived from stem cells of one monkey into the eyes of other monkeys without rejection and without the need for immunosuppressant drugs. Published in Stem Cell Reports, the study shows that this procedure is possible as long as a set of cells called the MHC are genetically matched between the host monkey and the new retinal cells.
A realistic hope of modern medicine is to replace damaged tissue with healthy cells grown in the lab. Currently, adult cells can be reprogrammed into stem cells, and then re-differentiated and grown into desired cell types. The researchers at RIKEN CDB led by Masayo Takahashi have already begun a clinical transplant trial in people with age-related macular degeneration. The team grew retinal pigment cells from induced pluripotent stem cells (iPSCs) and transplanted them into the damaged retina of a human participant. In order to avoid tissue rejection, they used autologous iPSCs—iPSCs that were created from the recipient’s own skin cells.
While this method is sound, producing autologous iPSCs is costly. Additionally, because the cells must grow at the same rate as they do during normal development, a person would have to wait more than a year before a transplant could be performed.
Notes lead author Sunao Sugita, “In order to make iPSC transplantation a practical reality, the current goal is to create banks of iPSC-derived tissues that can be transplanted into anyone as they are needed. However, immune responses and tissue rejection are big issues to overcome when transplanting tissue derived from other individuals.”
The new study tested a technique called MHC matching as a way to overcome this issue. Major histocompatibility complexes (MHCs) are a sets of cell-surface proteins found in all cells that function in the immune system. In humans, MHCs are also called human leukocyte antigens (HLAs). There are many genetic variations of MHCs, and after transplantation, if the MHCs of the transplanted cells are not recognized by the T cells of the host immune system, there is an immune response and the tissue is rejected.
To test whether MHC matching is a viable method, the team used retinal pigment cells that were grown from monkey iPSCs in the iPS cell bank at the Center for iPS Cell Research and Application, Kyoto University. They transplanted the cells into the subretinal space in monkeys with either genetically matched or non-matched MHCs.
The researchers found that these transplanted cells survived without rejection for at least 6 months in MHC-matched monkeys, without using any of the usually necessary immunosuppressant drugs. In contrast, rejection was relatively quick in the MHC-mismatched monkeys. Immunohistochemical examination showed that infiltration by inflammatory cells was only present in the transplanted grafts of MHC-mismatched monkeys. In vitro, the team saw that T cells failed to respond to the iPSC-derived retinal pigment cells if they were from an MHC-matched monkey.
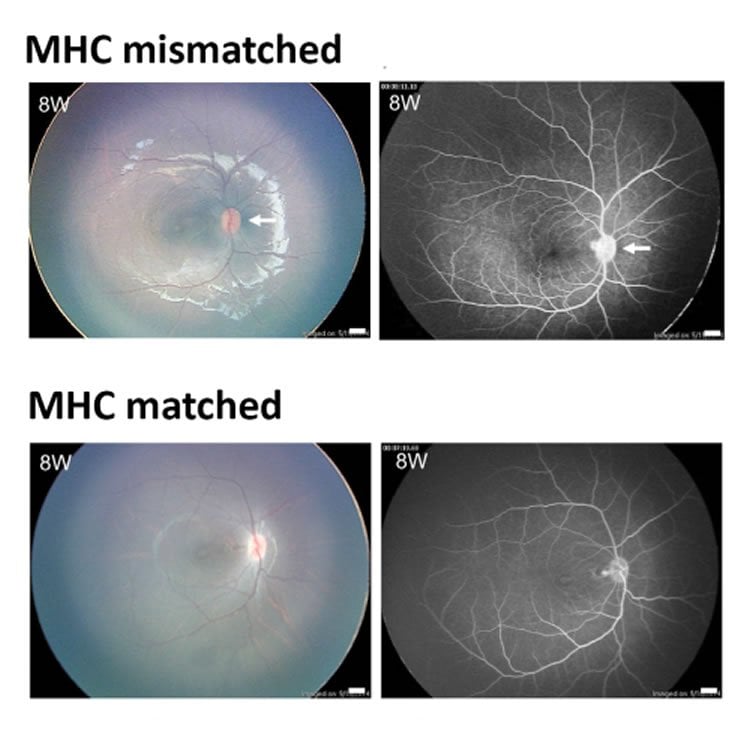
(B) Transplantation of monkey iPS-RPE cells into the subretinal space in an MHC-matched monkey. At 8 weeks (8W) after surgery, the results of color fundus photographs (upper left) and FA (upper right) revealed no inflammation. Throughout the 6-month observation period, there were no rejection signs in the subretinal space or the retina. Scale bars in color fundus and FA, 1.0 mm. NeuroscienceNews.com image is credited to the researchers.
In a separate study published in the same issue of Stem Cell Reports, the researchers saw similar results when they repeated this last experiment with human T cells and HLA-matched or unmatched retinal pigment cells grown from IPSCs.
Now that we have established the lack of immune response in monkeys and in human cells in vitro,” explains Sugita, “using the iPS cell bank appears to be a viable solution, at least in the case of retinal pigment epithelial cell transplantation.”
“In the next clinical trial,” continues Sugita, “we plan to use allogeneic iPS-retinal pigment epithelial cells from HLA homozygote donors. The clinical data after the transplantation will allow us to see if the iPS cell bank is truly useful or not. If so, I think this type of transplantation can become standard treatment within 5 years.”
Source: Adam Phillips – RIKEN
Image Source: This NeuroscienceNews.com image is credited to the researchers.
Original Research: Full open access research for “Lack of T Cell Response to iPSC-Derived Retinal Pigment Epithelial Cells from HLA Homozygous Donors” by Sunao Sugita, Yuko Iwasaki, Kenichi Makabe, Takafumi Kimura, Takaomi Futagami, Shinji Suegami, and Masayo Takahashi in Stem Cell Reports. Published online August 16 2016 doi:10.1016/j.stemcr.2016.08.011
Full open access research for “Successful Transplantation of Retinal Pigment Epithelial Cells from MHC Homozygote iPSCs in MHC-Matched Models” by Sunao Sugita, Yuko Iwasaki, Kenichi Makabe, Hiroyuki Kamao, Michiko Mandai, Takashi Shiina, Kazumasa Ogasawara, Yasuhiko Hirami, Yasuo Kurimoto, and Masayo Takahashi in Stem Cell Reports. Published online August 16 2016 doi:10.1016/j.stemcr.2016.08.010
[cbtabs][cbtab title=”MLA”]RIKEN. “Researchers Successfully Transplant iPSC Derived Retinal Cells from One Monkey to Another Without Rejection.” NeuroscienceNews. NeuroscienceNews, 23 September 2016.
<https://neurosciencenews.com/retinal-cell-transplant-5110/>.[/cbtab][cbtab title=”APA”]RIKEN. (2016, September 23). Researchers Successfully Transplant iPSC Derived Retinal Cells from One Monkey to Another Without Rejection. NeuroscienceNews. Retrieved September 23, 2016 from https://neurosciencenews.com/retinal-cell-transplant-5110/[/cbtab][cbtab title=”Chicago”]RIKEN. “Researchers Successfully Transplant iPSC Derived Retinal Cells from One Monkey to Another Without Rejection.” https://neurosciencenews.com/retinal-cell-transplant-5110/ (accessed September 23, 2016).[/cbtab][/cbtabs]
Abstract
Lack of T Cell Response to iPSC-Derived Retinal Pigment Epithelial Cells from HLA Homozygous Donors
Highlights
•We established human RPE cells from iPSCs in HLA homozygote donors
•iPS-RPE cells uniquely expressed HLA class I and class II molecules
•T lymphocytes responded to allogeneic iPS-RPE cells, but not iPSCs, in vitro
•T lymphocytes failed to respond to allogeneic iPS-RPE cells from HLA homozygote donors
Summary
Allografts of retinal pigment epithelial (RPE) cells have been considered for the treatment of ocular diseases. We recently started the transplantation of induced pluripotent stem cell (iPSC)-derived RPE cells for patients with age-related macular degeneration (autogenic grafts). However, there are at least two problems with this approach: (1) high cost, and (2) uselessness for acute patients. To resolve these issues, we established RPE cells from induced iPSCs in HLA homozygote donors. In vitro, human T cells directly recognized allogeneic iPSC-derived RPE cells that expressed HLA class I/II antigens. However, these T cells failed to respond to HLA-A, -B, and -DRB1-matched iPSC-derived RPE cells from HLA homozygous donors. Because of the lack of T cell response to iPSC-derived RPE cells from HLA homozygous donors, we can use these allogeneic iPSC-derived RPE cells in future clinical trials if the recipient and donor are HLA matched.
“Lack of T Cell Response to iPSC-Derived Retinal Pigment Epithelial Cells from HLA Homozygous Donors” by Sunao Sugita, Yuko Iwasaki, Kenichi Makabe, Takafumi Kimura, Takaomi Futagami, Shinji Suegami, and Masayo Takahashi in Stem Cell Reports. Published online August 16 2016 doi:10.1016/j.stemcr.2016.08.011
Abstract
Successful Transplantation of Retinal Pigment Epithelial Cells from MHC Homozygote iPSCs in MHC-Matched Models
Highlights
•We established RPE cells from iPSCs in MHC homozygote animals
•MHC-mismatching transplantation caused immune attacks of iPS-RPE allografts
•MHC-matching transplantation appeared to prevent the attacks of the allografts
•T cells responded to allogeneic iPS-RPE, but failed to respond to MHC homozygote RPE
Summary
There is an ongoing controversy as to whether major histocompatibility complex (MHC) matching is a solution for allogeneic stem cell transplantation. In the present study, we established retinal pigment epithelial (RPE) cells from induced pluripotent stem cells (iPSCs) in MHC homozygote donors. We observed no rejection signs in iPSC-derived RPE allografts of MHC-matched animal models without immunosuppression, whereas there were immune attacks around the graft and retinal tissue damage in MHC-mismatched models. In an immunohistochemical examination of MHC-mismatched allografts, the transplanted RPE sheets/cells were located in the subretinal space, but the RPE exhibited inflammatory and hypertrophic changes, and many inflammatory cells, e.g., Iba1+ cells, MHC class II+ cells, and CD3+ T cells, invaded the graft area. Conversely, these inflammatory cells poorly infiltrated the area around the transplanted retina if MHC-matched allografts were used. Thus, cells derived from MHC homozygous donors could be used to treat retinal diseases in histocompatible recipients.
“Successful Transplantation of Retinal Pigment Epithelial Cells from MHC Homozygote iPSCs in MHC-Matched Models” by Sunao Sugita, Yuko Iwasaki, Kenichi Makabe, Hiroyuki Kamao, Michiko Mandai, Takashi Shiina, Kazumasa Ogasawara, Yasuhiko Hirami, Yasuo Kurimoto, and Masayo Takahashi in Stem Cell Reports. Published online August 16 2016 doi:10.1016/j.stemcr.2016.08.010






