Summary: Researchers describe the mechanical forces at play during myelination.
Source: University at Buffalo.
Myelination depends on mechanical as well as chemical signaling and that may signal a new path to treatments for myelin diseases, such as MS.
Mechanical forces play a critical role in myelination, the formation of the protective coating that neurons need to function, researchers at the University at Buffalo have discovered.
The paper was published online June 6 in Nature Neuroscience.
The authors are scientists at UB’s Hunter James Kelly Research Institute (HJKRI), one of a handful of research institutes in the world with an exclusive focus on myelin and diseases of myelin, such as multiple sclerosis and leukodystrophies, and how they may be treated. The institute, part of UB’s New York State Center of Excellence in Bioinformatics and Life Sciences, was established in 1997 by Buffalo Bills Hall of Fame quarterback Jim Kelly and his wife, Jill, after their infant son, Hunter, was diagnosed with Krabbe Leukodystrophy, an inherited, fatal disorder. He died in 2005 at the age of 8.
The UB researchers found that Schwann cells, the cells that form myelin in the nervous system, respond to mechanical stimuli by activating certain molecules that are then transferred to the nucleus to trigger myelination.
“There were hints in previous studies that mechanical properties of tissues could influence the behavior of myelin-forming cells,” said senior author M. Laura Feltri, MD, professor of biochemistry and neurology in the Jacobs School of Medicine and Biomedical Sciences at UB and a researcher at HJKRI. “Our work proves for the first time, in vivo, that this is indeed the case. We have demonstrated that mechanical information is necessary for myelination to occur.”
The finding opens new possibilities in developing treatments for myelin-related diseases.
“Most medical treatments are based on altering chemical signals,” said Feltri. “This work says there is another level of control in myelin diseases that we can learn from and possibly exploit in the future. Potentially, in the future, we will be able to exploit a tissue’s mechanical properties, including the density, tension or elasticity of some part of the brain or peripheral nerves.”
Mechanical signaling is already well-accepted in the understanding of injury and repair in muscles and bones. “We know, for example, that after a bone fracture, one should put weight on the broken bone because this mechanical stimulation improves the formation of new bone cells,” she noted. “Now we know that a similar phenomenon is occurring with myelin cells.”
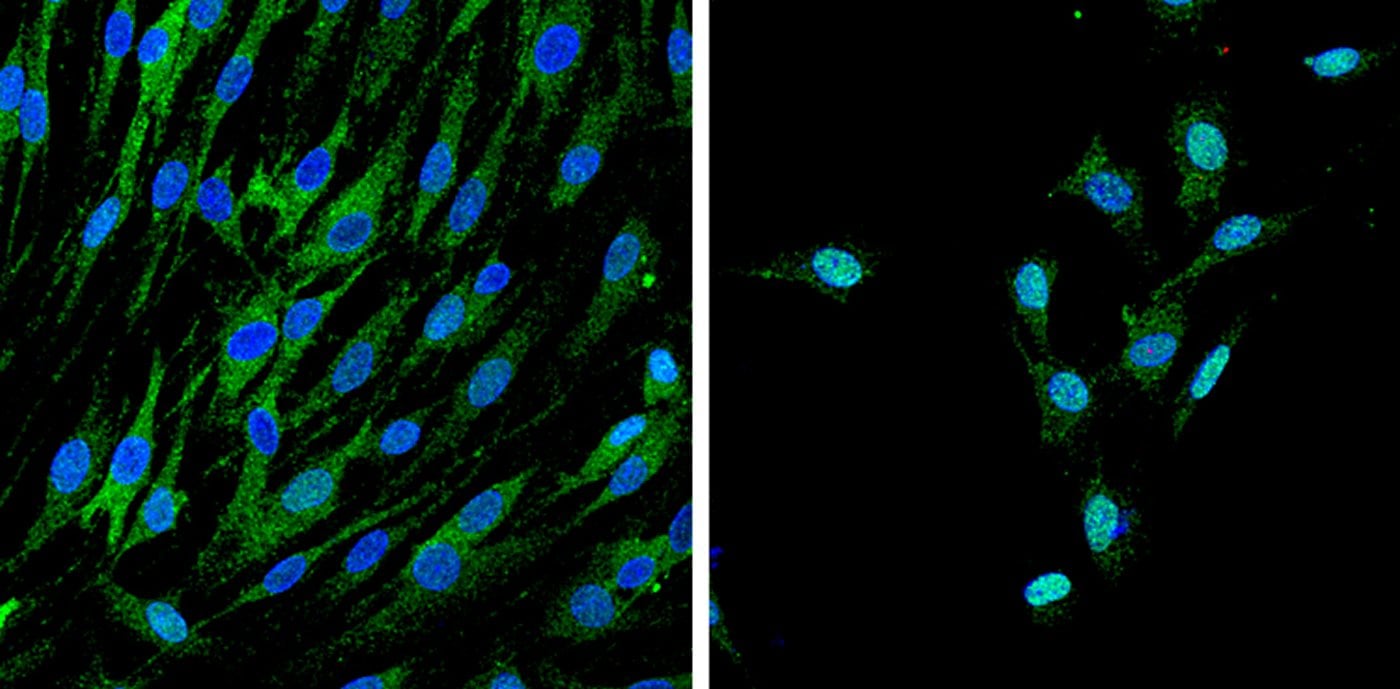
She added that similarly, traumatic brain or nerve injury causes mechanical stimulation of neural cells, and some hereditary neuropathies, such as hereditary neuropathy with liability to pressure palsies caused by deletion of certain genes, result in demyelination only after a compression injury. “Our work is beginning to shed light on these mechanisms.”
The paper also reported for the first time that myelination involves an important and evolutionarily conserved pathway called the Hippo signaling pathway. The Hippo pathway determines organ size through cell proliferation and cell death; it also has been implicated in some cancers.
The UB research focused on two genes that are controlled by the Hippo pathway: Yap and Taz, which, the scientists found, are required to make myelin and are activated by mechanical forces.When these genes were deleted in animal models, mice experienced severe peripheral neuropathy with symptoms, such as tremor, weakness and atrophy.
“That effect is caused by an impairment in an important developmental step that is necessary in order for myelin to be generated in the peripheral nervous system,” said Feltri.
Her research team will now focus on the physical properties of the nervous system and their effect on myelin formation and myelin diseases.
Yannick Poitelon, PhD, a senior postdoctoral fellow in Feltri’s laboratory, is first author. Co-authors at UB are Ruogang Zhao, PhD, assistant professor in the Department of Biomedical Engineering; Fraser Sim, PhD, assistant professor in the Department of Pharmacology and Toxicology; and Lawrence Wrabetz, MD, director of the HJKRI and professor in the departments of Neurology and Biochemistry.
Additional UB co-authors are K. Catignas, a biochemistry graduate student; D. Ameroso, a neuroscience graduate student; M. Palmisano, a graduate student from San Raffaele hospital in Italy; K. Abiko, a biochemistry undergraduate student and C. Berti, PhD, a senior research scientist in the Department of Neurology. Other UB co-authors are C. Williamson and Y. Hwang of the HJKRI and M. Asmani of the Department of Biomedical Engineering.
Other authors were from the University of Wisconsin-Madison and Mount Sinai Hospital in Toronto.
Funding: The research, a collaboration between the Jacobs School of Medicine and Biomedical Sciences and the School of Engineering and Applied Sciences, was funded by startup money to the HJKRI from New York State’s Empire State Development Corporation –Krabbe Disease Research Working Capital — and from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health.
Source: Ellen Goldbaum – University at Buffalo
Image Source: This NeuroscienceNews.com image is credited to Y. Poitelon, Feltri lab.
Original Research: Abstract for “YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells” by Yannick Poitelon, Camila Lopez-Anido, Kathleen Catignas, Caterina Berti, Marilena Palmisano, Courtney Williamson, Dominique Ameroso, Kansho Abiko, Yoonchan Hwang, Alex Gregorieff, Jeffrey L Wrana, Mohammadnabi Asmani, Ruogang Zhao, Fraser James Sim, Lawrence Wrabetz, John Svaren and Maria Laura Feltri in Nature Neuroscience. Published online June 6 2016 doi:10.1038/nn.4316
[cbtabs][cbtab title=”MLA”]University at Buffalo. “Myelin Cells Feel the Force.” NeuroscienceNews. NeuroscienceNews, 7 June 2016.
<https://neurosciencenews.com/myelination-ms-neuroscience-4404/>.[/cbtab][cbtab title=”APA”]University at Buffalo. (2016, June 7). Myelin Cells Feel the Force. NeuroscienceNews. Retrieved June 7, 2016 from https://neurosciencenews.com/myelination-ms-neuroscience-4404/[/cbtab][cbtab title=”Chicago”]University at Buffalo. “Myelin Cells Feel the Force.” https://neurosciencenews.com/myelination-ms-neuroscience-4404/ (accessed June 7, 2016).[/cbtab][/cbtabs]
Abstract
YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells
Myelination is essential for nervous system function. Schwann cells interact with neurons and the basal lamina to myelinate axons using known receptors, signals and transcription factors. In contrast, the transcriptional control of axonal sorting and the role of mechanotransduction in myelination are largely unknown. Yap and Taz are effectors of the Hippo pathway that integrate chemical and mechanical signals in cells. We describe a previously unknown role for the Hippo pathway in myelination. Using conditional mutagenesis in mice, we show that Taz is required in Schwann cells for radial sorting and myelination and that Yap is redundant with Taz. Yap and Taz are activated in Schwann cells by mechanical stimuli and regulate Schwann cell proliferation and transcription of basal lamina receptor genes, both necessary for radial sorting of axons and subsequent myelination. These data link transcriptional effectors of the Hippo pathway and of mechanotransduction to myelin formation in Schwann cells.
“YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells” by Yannick Poitelon, Camila Lopez-Anido, Kathleen Catignas, Caterina Berti, Marilena Palmisano, Courtney Williamson, Dominique Ameroso, Kansho Abiko, Yoonchan Hwang, Alex Gregorieff, Jeffrey L Wrana, Mohammadnabi Asmani, Ruogang Zhao, Fraser James Sim, Lawrence Wrabetz, John Svaren and Maria Laura Feltri in Nature Neuroscience. Published online June 6 2016 doi:10.1038/nn.4316