Summary: A new study strenghtens the argument that some forms of multiple sclerosis are genetic.
Source: University of British Columbia.
Discovery helps erase doubts that some forms of MS are inherited.
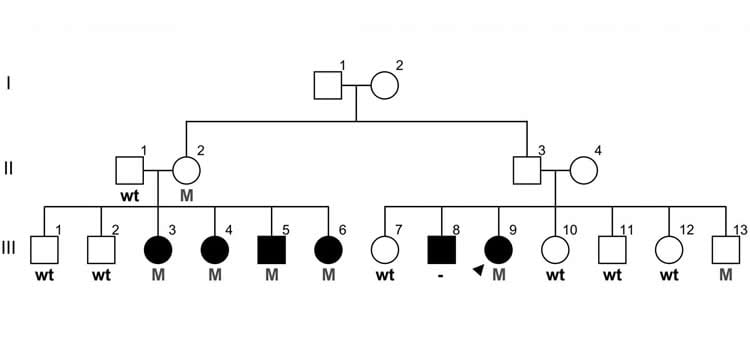
Less than a year after publishing research identifying a single genetic mutation that caused multiple sclerosis (MS) in two Canadian families, scientists at the University of British Columbia have found a combination of two other mutations in another family that made them highly susceptible to the disease.
The “double gene” mutation was identified in a large Canadian family with five members diagnosed with MS – all of whom had the DNA abnormality. Two other family members had the same mutation but didn’t develop MS, indicating that some other genetic or environmental conditions are still necessary to trigger the disease process.
The discovery of this mutation, on top of last year’s findings, should help erase doubts that at least some forms of MS are inherited. The prevailing view has been that a combination of many genetic variations causes a slight increase in susceptibility. In this family, individuals with the double gene mutation have about a 7-in-10 chance of developing MS, compared to a 1-in-1,000 risk in the general population.
These mutations, described in the journal Human Mutation, impair both immune function and phagocytosis, the process by which cells eliminate debris and pathogens.
“This is the first time that problems with phagocytosis have been linked to MS, and provides scientists with a better understanding the disease’s origins and targets for developing new treatments,” said lead author Carles Vilarino-Guell, an Assistant Professor of Medical Genetics who collaborated with colleagues at Australia’s Florey Institute of Neuroscience and Mental Health.
The findings also could be used to screen people with a family history of the disease; an individual who was found to have this mutation could be a candidate for early diagnostic imaging long before symptoms appear, or could opt to reduce environmental risks by taking Vitamin D supplements or quitting smoking.
MS results from the body’s immune system attacking myelin, the fatty material that insulates neurons and enables rapid transmission of electrical signals. When myelin is damaged, communication between the brain and other parts of the body is disrupted, leading to vision problems, muscle weakness, difficulty with balance and coordination, and cognitive impairments. Canada has one of the highest rate of MS in the world, for reasons that elude scientists.
The double mutation, unlike the single mutation described last year, leads to the more typical “relapsing-remitting” form of MS, in which the symptoms come and go. These differences in clinical symptoms suggests that different biological processes are responsible for each type of MS, which could explain why treatments for relapsing-remitting patients are ineffective for people with more debilitating, progressive form of the disease.
The family with this mutation had donated to a Canadian-wide collection of blood samples from people with MS, begun in 1993 by co-author A. Dessa Sadovnick, a UBC Professor of Medical Genetics and Neurology. The 20-year project, funded by the MS Society of Canada and the Multiple Sclerosis Scientific Research Foundation, has samples from 4,400 people with MS, plus 8,600 blood relatives – one of the largest such biobanks in the world, stored at UBC and Vancouver Coastal Health’s Djavad Mowafaghian Centre for Brain Health.
Source: Brian Kladko – University of British Columbia
Image Source: NeuroscienceNews.com image is credited to Carles Vilarino-Guell/University of British Columbia.
Original Research: Abstract for “Purinergic receptors P2RX4 and P2RX7 in familial multiple sclerosis” by A Dessa Sadovnick, Ben J Gu, Anthony L Traboulsee, Cecily Q Bernales, Mary Encarnacion, Irene M Yee, Maria G Criscuoli, Xin Huang, Amber Ou, Carol J Milligan, Steven Petrou, James S Wiley, and Carles Vilariño-Güell in Human Mutation. Published online April 13 2017 doi:10.1002/humu.23218
[cbtabs][cbtab title=”MLA”]University of British Columbia “More Multiple Sclerosis Causing Mutations Found in Canadian Families.” NeuroscienceNews. NeuroscienceNews, 17 April 2017.
<https://neurosciencenews.com/multiple-sclerosis-mutations-6430/>.[/cbtab][cbtab title=”APA”]University of British Columbia (2017, April 17). More Multiple Sclerosis Causing Mutations Found in Canadian Families. NeuroscienceNew. Retrieved April 17, 2017 from https://neurosciencenews.com/multiple-sclerosis-mutations-6430/[/cbtab][cbtab title=”Chicago”]University of British Columbia “More Multiple Sclerosis Causing Mutations Found in Canadian Families.” https://neurosciencenews.com/multiple-sclerosis-mutations-6430/ (accessed April 17, 2017).[/cbtab][/cbtabs]
Abstract
Purinergic receptors P2RX4 and P2RX7 in familial multiple sclerosis
Genetic variants in the purinergic receptors P2RX4 and P2RX7 have been shown to affect susceptibility to multiple sclerosis (MS). In this study, we set out to evaluate whether rare coding variants of major effect could also be identified in these purinergic receptors. Sequencing analysis of P2RX4 and P2RX7 in 193 MS patients and 100 controls led to the identification of a rare three variant haplotype (P2RX7 rs140915863:C>T [p.T205M], P2RX7 rs201921967:A>G [p.N361S], and P2RX4 rs765866317:G>A [p.G135S]) segregating with disease in a multi-incident family with six family members diagnosed with MS (logarithm of odds = 3.07). Functional analysis of this haplotype in HEK293 cells revealed impaired P2X7 surface expression (P < 0.01), resulting in over 95% inhibition of adenosine triphosphate (ATP)-induced pore function (P < 0.001) and a marked reduction in phagocytic ability (P < 0.05). In addition, transfected cells showed 40% increased peak ATP-induced inward current (P < 0.01), and a greater Ca2+ response to the P2X4 135S variant compared with wild type (P < 0.0001). Our study nominates rare genetic variants in P2RX4 and P2RX7 as major genetic contributors to disease, further supporting a role for these purinergic receptors in MS and the disruption of transmembrane cation channels leading to impairment of phagocytosis as the pathological mechanisms of disease.
“Purinergic receptors P2RX4 and P2RX7 in familial multiple sclerosis” by A Dessa Sadovnick, Ben J Gu, Anthony L Traboulsee, Cecily Q Bernales, Mary Encarnacion, Irene M Yee, Maria G Criscuoli, Xin Huang, Amber Ou, Carol J Milligan, Steven Petrou, James S Wiley, and Carles Vilariño-Güell in Human Mutation. Published online April 13 2017 doi:10.1002/humu.23218






