Summary: Clinical trials show that a new drug called ocrelizumab can reduce attacks in people with RRMS, and can reduce new symptom development in patients with primary progressive multiple sclerosis.
Source: McGill University.
Drug shown to reduce new attacks/symptom progression in some patients.
In separate clinical trials, a drug called ocrelizumab has been shown to reduce new attacks in patients with relapsing remitting multiple sclerosis (MS), and new symptom progression in primary progressive MS.
Three studies conducted by an international team of researchers, which included Amit Bar-Or and Douglas Arnold from the Montreal Neurological Institute and Hospital of McGill University, have discovered that ocrelizumab can significantly reduce new attacks in patients with relapsing MS, as well as slow the progression of symptoms caused by primary progressive MS.
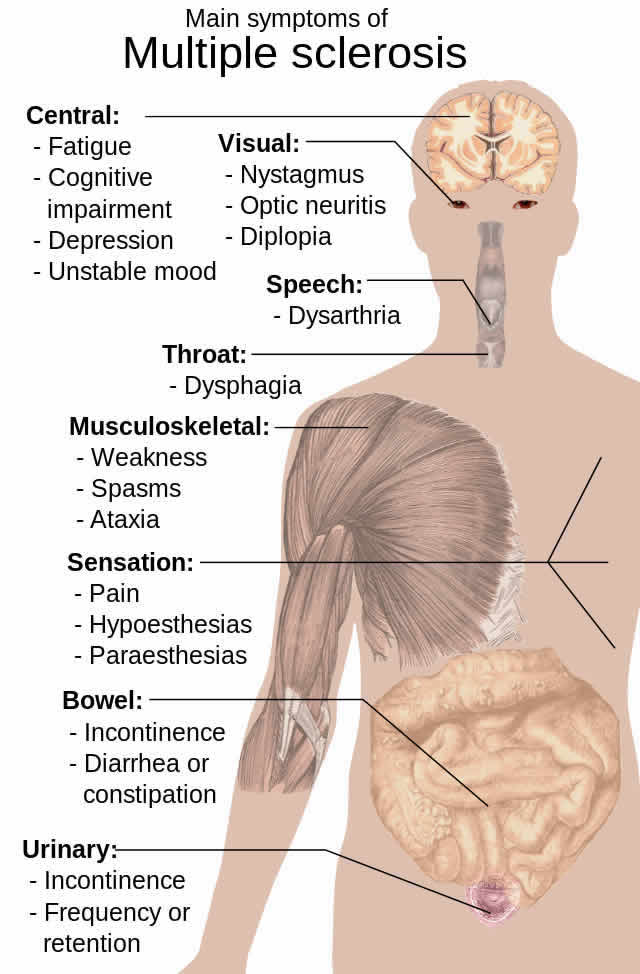
In one study, 732 patients with primary progressive MS were randomized on a 2:1 ratio to receive either ocrelizumab, a humanized monoclonal antibody that depletes CD20+ B cells, or a placebo.
The proportion of patients with 12-week confirmed disability progression was 39.3 per cent with the placebo versus 32.9 per cent with ocrelizumab. After 24 weeks, the proportion with confirmed disability progression was 35.7 per cent with placebo versus 29.6 per cent with ocrelizumab. By week 120, timed 25-foot walk worsened by 55.1 per cent for placebo versus 38.9 per cent for ocrelizumab.
Patients given ocrelizumab were also found to have fewer new brain lesions and less brain volume loss than those given the placebo.
Researchers also tested ocrelizumab in two separate studies of patients with relapsing remitting MS, one a group of 821 and the other 835. In both studies, patients were randomized on a 1:1 ratio to receive either ocrelizumab or an already established treatment for relapsing MS: subcutaneous interferon-beta, injected three times weekly. Compared to the placebo, relapse rates in patients given ocrelizumab were 46-per-cent lower in one study and 47-per-cent lower in the other. Ocrelizumab was found to reduce the risk of disability progression after 12 weeks and 24 weeks, and reduced the number of new brain lesions.
The study noted that infusion-related reactions occurred in 34.3 per cent of ocrelizumab-treated patients. Serious infections were not more frequent with ocrelizumab compared to the interferon (1.3 versus 2.9 per cent respectively). Malignancies occurred in four ocrelizumab-treated patients and in two interferon-treated patients. Further observation is required to determine the long-term safety of ocrelizumab.
“The results in patients with relapsing remitting MS not only demonstrate very high efficacy against relapses, but also underscore the important emerging role of B cells of the immune system in the development of relapses,” says Bar-Or. “While the results in patients with primary progressive MS are more modest, they nonetheless represent the very first successful trial in such patients, a breakthrough as primary progressive MS now transitions from a previously untreatable condition to one that can be impacted by therapy. It is an important step forward in the field.”
Funding: These studies were funded by Roche.
Source: Shawn Hayward – McGill University
Image Source: NeuroscienceNews.com image is in the public domain.
Original Research: Full open access research for “Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis” by Xavier Montalban, M.D., Stephen L. Hauser, M.D., Ludwig Kappos, M.D., Douglas L. Arnold, M.D., Amit Bar-Or, M.D., Giancarlo Comi, M.D., Jérôme de Seze, M.D., Gavin Giovannoni, M.D., Hans-Peter Hartung, M.D., Bernhard Hemmer, M.D., Fred Lublin, M.D., Kottil W. Rammohan, M.D., Krzysztof Selmaj, M.D., Anthony Traboulsee, M.D., Annette Sauter, Ph.D., Donna Masterman, M.D., Paulo Fontoura, M.D., Ph.D., Shibeshih Belachew, M.D., Ph.D., Hideki Garren, M.D., Ph.D., Nicole Mairon, M.D., Peter Chin, M.D., and Jerry S. Wolinsky, M.D., for the ORATORIO Clinical Investigators in New England Journal of Medicine. Published online December 21 2016 doi:0.1056/NEJMoa1606468
[cbtabs][cbtab title=”MLA”]McGill University “Breakthrough in Multiple Sclerosis Treatment.” NeuroscienceNews. NeuroscienceNews, 9 January 2017.
<https://neurosciencenews.com/ms-treatment-breakthrough-5895/>.[/cbtab][cbtab title=”APA”]McGill University (2017, January 9). Breakthrough in Multiple Sclerosis Treatment. NeuroscienceNew. Retrieved January 9, 2017 from https://neurosciencenews.com/ms-treatment-breakthrough-5895/[/cbtab][cbtab title=”Chicago”]McGill University “Breakthrough in Multiple Sclerosis Treatment.” https://neurosciencenews.com/ms-treatment-breakthrough-5895/ (accessed January 9, 2017).[/cbtab][/cbtabs]
Abstract
Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis
BACKGROUND
An evolving understanding of the immunopathogenesis of multiple sclerosis suggests that depleting B cells could be useful for treatment. We studied ocrelizumab, a humanized monoclonal antibody that selectively depletes CD20-expressing B cells, in the primary progressive form of the disease.
METHODS
In this phase 3 trial, we randomly assigned 732 patients with primary progressive multiple sclerosis in a 2:1 ratio to receive intravenous ocrelizumab (600 mg) or placebo every 24 weeks for at least 120 weeks and until a prespecified number of confirmed disability progression events had occurred. The primary end point was the percentage of patients with disability progression confirmed at 12 weeks in a time-to-event analysis.
RESULTS
The percentage of patients with 12-week confirmed disability progression was 32.9% with ocrelizumab versus 39.3% with placebo (hazard ratio, 0.76; 95% confidence interval [CI], 0.59 to 0.98; P=0.03). The percentage of patients with 24-week confirmed disability progression was 29.6% with ocrelizumab versus 35.7% with placebo (hazard ratio, 0.75; 95% CI, 0.58 to 0.98; P=0.04). By week 120, performance on the timed 25-foot walk worsened by 38.9% with ocrelizumab versus 55.1% with placebo (P=0.04); the total volume of brain lesions on T2-weighted magnetic resonance imaging (MRI) decreased by 3.4% with ocrelizumab and increased by 7.4% with placebo (P<0.001); and the percentage of brain-volume loss was 0.90% with ocrelizumab versus 1.09% with placebo (P=0.02). There was no significant difference in the change in the Physical Component Summary score of the 36-Item Short-Form Health Survey. Infusion-related reactions, upper respiratory tract infections, and oral herpes infections were more frequent with ocrelizumab than with placebo. Neoplasms occurred in 2.3% of patients who received ocrelizumab and in 0.8% of patients who received placebo; there was no clinically significant difference between groups in the rates of serious adverse events and serious infections.
CONCLUSIONS
Among patients with primary progressive multiple sclerosis, ocrelizumab was associated with lower rates of clinical and MRI progression than placebo. Extended observation is required to determine the long-term safety and efficacy of ocrelizumab. (Funded by F. Hoffmann–La Roche; ORATORIO ClinicalTrials.gov number, NCT01194570.)
“Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis” by Xavier Montalban, M.D., Stephen L. Hauser, M.D., Ludwig Kappos, M.D., Douglas L. Arnold, M.D., Amit Bar-Or, M.D., Giancarlo Comi, M.D., Jérôme de Seze, M.D., Gavin Giovannoni, M.D., Hans-Peter Hartung, M.D., Bernhard Hemmer, M.D., Fred Lublin, M.D., Kottil W. Rammohan, M.D., Krzysztof Selmaj, M.D., Anthony Traboulsee, M.D., Annette Sauter, Ph.D., Donna Masterman, M.D., Paulo Fontoura, M.D., Ph.D., Shibeshih Belachew, M.D., Ph.D., Hideki Garren, M.D., Ph.D., Nicole Mairon, M.D., Peter Chin, M.D., and Jerry S. Wolinsky, M.D., for the ORATORIO Clinical Investigators in New England Journal of Medicine. Published online December 21 2016 doi:0.1056/NEJMoa1606468






