Summary: Converting skin cells to functional brain cells relies on precise insertion of Line-1 retrotransposons. These L1 insertions correlate with open chromatin and lncRNA transcription.
Source: King Abdullah University of Science and Technology
The process of making functional brain cells in a lab dish requires the precise activation of selfish genetic elements known as LINE-1 (L1) retrotransposons. The finding, from researchers at KAUST, could lead to safer and more effective regenerative therapies for Parkinson’s disease and other brain conditions.
The genomes of humans, mice and other mammals have hundreds of thousands of L1 elements. Most are inactive, yet some retain the ability to make copies of themselves and jump into different segments of DNA, with impacts on gene regulation that can be both harmful and beneficial. Sometimes, the jumping genes can trigger disease. In early brain development, however, L1 activity is needed for neurons to form properly–although the reason had been unclear.
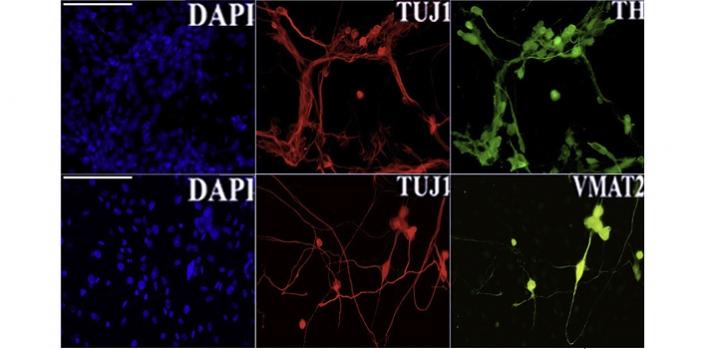
Image 1: Reprogramming efficiency and cell conversion were confirmed by an immunofluorescent assay for principal neural commitment (TH, TUJ1 and VMAT2) and dopaminergic neuron markers (TH).
Image 2: Engineered skin cells (red) become dopamine-producing neurons (green).
Reproduced from reference one under a creative commons license © 2020 Della Valle et al.
To address this question, Valerio Orlando and colleagues turned to a cellular model of neuronal development. Working with scientists in Italy, they engineered skin cells taken from mouse embryos to express various “reprogramming factors'” that converted them into dopamine-producing neurons, similar to those found in the substantia nigra, a small structure located deep in the brain. In the process, the researchers observed L1 activation.
They treated the cells with two kinds of drugs that block L1 dynamics. Both treatments dramatically impaired the efficiency of cell conversion, demonstrating that “activation is required for successful reprogramming of skin cells into neuronal cells,” according to Francesco Della Valle, a postdoc in Orlando’s lab group, and the first author of the new study.
The researchers then sequenced all the DNA inside the cells, both before and after their conversion, to determine where the L1 elements had newly inserted themselves into the genome. They found insertional hotspots around the genes involved in neuronal lineage commitment and neuron function. Consequently, the DNA at these sites was less densely packaged, allowing for higher levels of relevant gene expression.
“Our work boosts the concept that repetitive elements play an important, unprecedented role in cell differentiation and tissue specific developmental programs,” Della Valle says.
Those insights could prove invaluable as researchers design new kinds of cell therapies to replace the dopamine-producing neurons lost in people with Parkinson’s disease and related disorders. “Aberrant L1 activity could threaten the viability or safety of any such product,” notes Della Valle, “while optimizing L1 function could enhance the manufacturing and consistency of this type of regenerative treatment.”
Source:
King Abdullah University of Science and Technology
Media Contact:
Carolyn Unck
Image Source:
Image 1: Reprogramming efficiency and cell conversion were confirmed by an immunofluorescent assay for principal neural commitment (TH, TUJ1 and VMAT2) and dopaminergic neuron markers (TH).
Image 2: Engineered skin cells (red) become dopamine-producing neurons (green).
Reproduced from reference one under a creative commons license © 2020 Della Valle et al.
Original Research:
Della Vale, F., Thimma, M.P., Caiazzo, M., Pulcrano, S., Celii, M., Adroub, S.A., Liu, P., Lobato, G.A., Broccoli, V. & Orlando, V. Transdifferentiation of mouse embryonic fibroblasts into dopaminergic neurons reactivates LINE-1 repetitive elements. Stem Cell Reports 14, 60-74 (2020).
http://dx.doi.org/10.1016/j.stemcr.2019.12.002
Highlights
• L1 activation accompanies induced dopaminergic neuron maturation
• L1 inhibition impairs the transdifferentiation potential of MEFs
• L1 retrotransposition creates a lineage-specific genetic mosaicism
• L1 insertions correlates with open chromatin and lncRNA transcription
Summary
In mammals, LINE-1 (L1) retrotransposons constitute between 15% and 20% of the genome. Although only a few copies have retained the ability to retrotranspose, evidence in brain and differentiating pluripotent cells indicates that L1 retrotransposition occurs and creates mosaics in normal somatic tissues. The function of de novo insertions remains to be understood. The transdifferentiation of mouse embryonic fibroblasts to dopaminergic neuronal fate provides a suitable model for studying L1 dynamics in a defined genomic and unaltered epigenomic background. We found that L1 elements are specifically re-expressed and mobilized during the initial stages of reprogramming and that their insertions into specific acceptor loci coincides with higher chromatin accessibility and creation of new transcribed units. Those events accompany the maturation of neuronal committed cells. We conclude that L1 retrotransposition is a non-random process correlating with chromatin opening and lncRNA production that accompanies direct somatic cell reprogramming.