Summary: A new study reveals elevated glial activation in the brains of those with fibromyalgia.
Source: Mass General.
A study by Massachusetts General Hospital (MGH) researchers – collaborating with a team at the Karolinska Institutet in Sweden – has documented for the first time widespread inflammation in the brains of patients with the poorly understood condition called fibromyalgia. Their report has been published online in the journal Brain, Behavior and Immunity.
“We don’t have good treatment options for fibromyalgia, so identifying a potential treatment target could lead to the development of innovative, more effective therapies,” says Marco Loggia, PhD, of the MGH-based Martinos Center for Biomedical Imaging, co-senior author of the report. “And finding objective neurochemical changes in the brains of patients with fibromyalgia should help reduce the persistent stigma that many patients face, often being told their symptoms are imaginary and there’s nothing really wrong with them.”
Characterized by symptoms including chronic widespread pain, sleep problems, fatigue, and problems with thinking and memory, fibromyalgia affects around 4 million adults in the U.S., according to the Centers for Disease Control and Prevention. Previous research from the Karolinska group led by Eva Kosek, MD, PhD, co-senior author of the current study, suggested a potential role for neuroinflammation in the condition – including elevated levels of inflammatory proteins in the cerebrospinal fluid – but no previous study has directly visualized neuroinflammation in fibromyalgia patients.
A 2015 study by Loggia’s team used combined MR/PET scanning to document neuroinflammation – specifically activation of glial cells – in the brains of patients with chronic back pain. Hypothesizing that similar glial activation might be found in fibromyalgia patients as well, his team used the same PET radiopharmaceutical, which binds to the translocator protein (TSPO) that is overexpressed by activated glial cells, in their study enrolling 20 fibromyalgia patients and 14 control volunteers.
At the same time, Kosek’s team at Karolinska had enrolled a group of 11 patients and an equal number of control participants for a similar study with the TSPO-binding PET tracer. Since that radiopharmaceutical binds to two types of glial cells – microglia and astrocytes – they also imaged 11 patients, 6 who had the TSPO imaging and 5 others, and another 11 controls with a PET tracer that is thought to bind preferentially to astrocytes and not to microglia. At both centers, participants with fibromyalgia completed questionnaires to assess their symptoms. When the MGH team became aware of the similar investigation the Karolinska group had underway, the teams decided to combine their data into a single study.
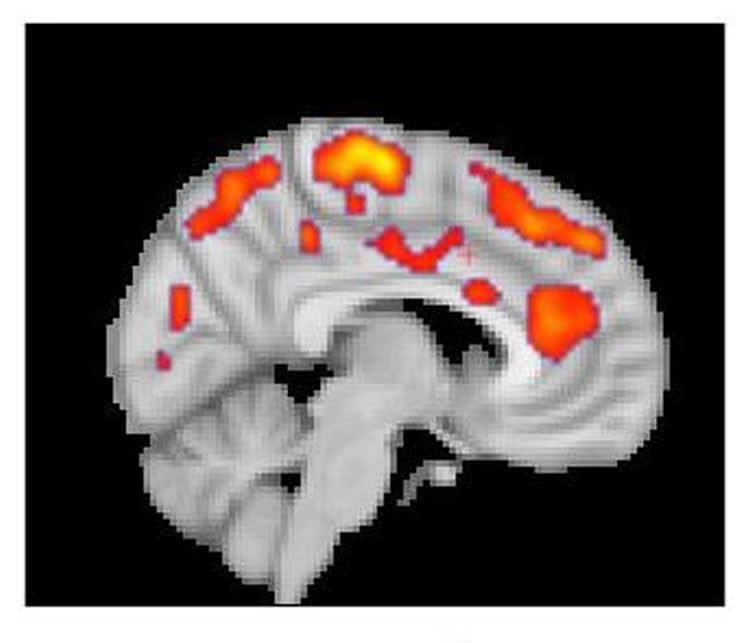
The results from both centers found that glial activation in several regions of the brains of fibromyalgia patients was significantly greater than it was in control participants. Compared to the MGH team’s chronic back pain study, TSPO elevations were more widespread throughout the brain, which Loggia indicates corresponds to the more complex symptom patterns of fibromyalgia. TSPO levels in a structure called the cingulate gyrus – an area associated with emotional processing where neuroinflammation has been reported in patients with chronic fatigue syndrome – corresponded with patients reported levels of fatigue. The Karolinska team’s studies with the astrocyte-binding tracer found little difference between patients and controls, suggesting that microglia were primarily responsible for the increased neuro-inflammation in fibromyalgia patients.
“The activation of glial cells we observed in our studies releases inflammatory mediators that are thought to sensitize pain pathways and contribute to symptoms such as fatigue,” says Loggia, an assistant professor of Radiology at Harvard Medical School. “The ability to join forces with our colleagues at Karolinska was fantastic, because combining our data and seeing similar results at both sites gives confidence to the reliability of our results.”
The co-lead authors of the Brain, Behavior and Immunity report are Daniel Albrecht, PhD, MGH Martinos Center and Department of Radiology, and Anton Forsberg, PhD, Karolinska Institutet.
Funding: Support for the study includes U.S. Department of Defense grant W81XWH-14-1-0543; National Institutes of Health grants R01 NS094306-01A1, R01 NS095937-01A1 and R21 NS087472-01A1; an International Association for the Study of Pain Early Career Award, and funding from the Stockholm County Council the Swedish Research Council, the Swedish Rheumatism Association and the Fibromyalgia Association of Sweden.
Source: Terri Ogan – Mass General
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Marco Loggia, PhD, Martinos Center for Biomedical Imaging, Massachusetts General Hospital.
Original Research: Open access research for “Brain glial activation in fibromyalgia – A multi-site positron emission tomography investigation” by Daniel S.Albrecht, Anton Forsberg, Angelica Sandström, Courtney Bergan, Diana Kadetoff, Ekaterina Protsenko, Jon Lamp, Yvonne C. Lee, Caroline Olgart Höglund, Ciprian Catana, Simon Cervenka, Oluwaseun Akeju, Mats Lekander, George Cohen, Christer Halldin, Norman Taylor, Minhae Kim, Jacob M. Hooker, Robert R. Edwards, Vitaly Napadowa, Eva Kosek, and Marco L.Loggia in Brain, Behavior and Immunity. Published September 14 2018.
doi:10.1016/j.bbi.2018.09.018
[cbtabs][cbtab title=”MLA”]Mass General”Widespread Inflammation in Brains of Those with Fibromyalgia.” NeuroscienceNews. NeuroscienceNews, 27 September 2018.
<https://neurosciencenews.com/inflammation-fibromyalgia-9925/>.[/cbtab][cbtab title=”APA”]Mass General(2018, September 27). Widespread Inflammation in Brains of Those with Fibromyalgia. NeuroscienceNews. Retrieved September 27, 2018 from https://neurosciencenews.com/inflammation-fibromyalgia-9925/[/cbtab][cbtab title=”Chicago”]Mass General”Widespread Inflammation in Brains of Those with Fibromyalgia.” https://neurosciencenews.com/inflammation-fibromyalgia-9925/ (accessed September 27, 2018).[/cbtab][/cbtabs]
Abstract
Brain glial activation in fibromyalgia – A multi-site positron emission tomography investigation
Fibromyalgia (FM) is a poorly understood chronic condition characterized by widespread musculoskeletal pain, fatigue, and cognitive difficulties. While mounting evidence suggests a role for neuroinflammation, no study has directly provided evidence of brain glial activation in FM. In this study, we conducted a Positron Emission Tomography (PET) study using [11C]PBR28, which binds to the translocator protein (TSPO), a protein upregulated in activated microglia and astrocytes. To enhance statistical power and generalizability, we combined datasets collected independently at two separate institutions (Massachusetts General Hospital [MGH] and Karolinska Institutet [KI]). In an attempt to disentangle the contributions of different glial cell types to FM, a smaller sample was scanned at KI with [11C]-L-deprenyl-D2 PET, thought to primarily reflect astrocytic (but not microglial) signal.
Thirty-one FM patients and 27 healthy controls (HC) were examined using [11C]PBR28 PET. 11 FM patients and 11 HC were scanned using [11C]-L-deprenyl-D2 PET. Standardized uptake values normalized by occipital cortex signal (SUVR) and distribution volume (VT) were computed from the [11C]PBR28 data. [11C]-L-deprenyl-D2 was quantified using λ k3. PET imaging metrics were compared across groups, and when differing across groups, against clinical variables.
Compared to HC, FM patients demonstrated widespread cortical elevations, and no decreases, in [11C]PBR28 VT and SUVR, most pronounced in the medial and lateral walls of the frontal and parietal lobes. No regions showed significant group differences in [11C]-L-deprenyl-D2 signal, including those demonstrating elevated [11C]PBR28 signal in patients (p’s ≥ 0.53, uncorrected). The elevations in [11C]PBR28 VT and SUVR were correlated both spatially (i.e., were observed in overlapping regions) and, in several areas, also in terms of magnitude. In exploratory, uncorrected analyses, higher subjective ratings of fatigue in FM patients were associated with higher [11C]PBR28 SUVR in the anterior and posterior middle cingulate cortices (p’s < 0.03). SUVR was not significantly associated with any other clinical variable. Our work provides the first in vivo evidence supporting a role for glial activation in FM pathophysiology. Given that the elevations in [11C]PBR28 signal were not also accompanied by increased [11C]-L-deprenyl-D2 signal, our data suggests that microglia, but not astrocytes, may be driving the TSPO elevation in these regions. Although [11C]-L-deprenyl-D2 signal was not found to be increased in FM patients, larger studies are needed to further assess the role of possible astrocytic contributions in FM. Overall, our data support glial modulation as a potential therapeutic strategy for FM.






