Summary: Findings may provide clues that may help researchers develop new techniques to regenerate hair cells in the inner ear and reverse hearing loss.
Source: Oregon Health & Science University
New research reveals a key insight into the development of hair bundles, the intricately complex assemblies in the inner ear responsible for hearing.
Hair bundles are precisely arranged cellular structures deep within the spiral cavity of the inner ear. Together, they convert vibrational energy into electrical signals in the brain that translate into the sensation of hearing. Once they’re lost – whether by loud noise, toxins, disease or aging – they do not naturally regenerate in people and other mammals.
The new research led by scientists at Oregon Health & Science University provides important clues that may help scientists develop new techniques to regenerate hair cells and reverse hearing loss.
In the study published today in the journal Current Biology, researchers discovered the development of hair bundles occurs in a kind of feedback loop in which form follows function and function drives form.
Using mice, which closely model human hearing, the researchers found stereocilia, roughly 100 of which are assembled into a hair bundle, widened simultaneously with the onset of mechanotransduction – the action of converting mechanical signals in the form of sound into electrical signals measured within the brain. The stereocilia only elongated to their mature lengths after transduction had been established.
It turns out that form and function are mutually reinforcing.
“We’ve been looking at these as separate pathways,” said lead author Jocelyn Krey, Ph.D., staff scientist in the Oregon Hearing Research Center and the Vollum Institute at OHSU. “But in the course of this research, we observed the change in form occurs at the same time as the conversion of mechanical to electrical signals. So we’re seeing these happen together, and feeding each other in a way we hadn’t seen before.”
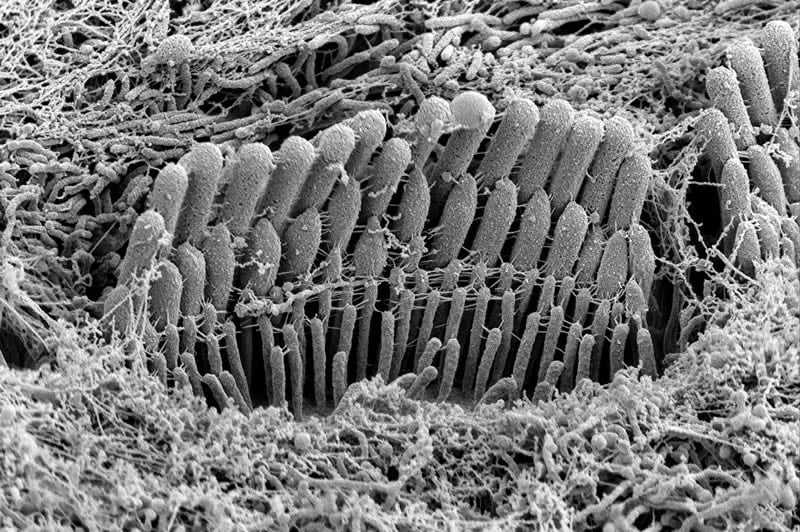
The researchers discovered when they examined mice lacking transduction or used a compound to block transduction, the animals did not develop the classic staircase-shaped form of mature hair bundles.
Researchers say the study suggests that new techniques to reverse hearing loss should focus on the critical importance of early development.
“In the future, with the rapid development of gene editing tools like CRISPR, we will be able to turn on genes at will,” said senior author Peter Barr-Gillespie, Ph.D., professor in the Oregon Hearing Research Center and senior scientist in the Vollum Institute. “I have no doubt we will be there in 5 or 10 years.”
Barr-Gillespie also serves as OHSU’s chief research officer and executive vice president.
Funding: The study was supported by confocal microscopy conducted in the OHSU Advanced Light Microscopy Core through National Institutes of Health grant P30 NS061800; electron microscopy performed at the OHSU Multiscale Microscopy Core; and NIH grants R03 DC014544; R01 DC014720; R01 DC002368 and R01 DC011034.
Source:
Oregon Health & Science University
Media Contacts:
Erik Robinson – Oregon Health & Science University
Image Source:
The image is credited to OHSU.
Original Research: Open access
“Mechanotransduction-Dependent Control of Stereocilia Dimensions and Row Identity in Inner Hair Cells”. Jocelyn Krey et al.
Current Biology doi:10.1016/j.cub.2019.11.076.
Abstract
Mechanotransduction-Dependent Control of Stereocilia Dimensions and Row Identity in Inner Hair Cellss
Highlights
• Widening of cochlear stereocilia correlates with acquisition of transduction
• Mouse mutants lacking transduction have stereocilia with altered dimensions
• Transduction mutants have altered distribution of row 1 and 2 marker proteins
• Blocking transduction channels with tubocurarine phenocopies transduction mutants
Summary
Actin-rich structures, like stereocilia and microvilli, are assembled with precise control of length, diameter, and relative spacing. By quantifying actin-core dimensions of stereocilia from phalloidin-labeled mouse cochleas, we demonstrated that inner hair cell stereocilia developed in specific stages, where a widening phase is sandwiched between two lengthening phases. Moreover, widening of the second-tallest stereocilia rank (row 2) occurred simultaneously with the appearance of mechanotransduction. Correspondingly, Tmc1KO/KO;Tmc2KO/KO or TmieKO/KO hair cells, which lack transduction, have significantly altered stereocilia lengths and diameters, including a narrowed row 2. EPS8 and the short splice isoform of MYO15A, identity markers for mature row 1 (the tallest row), lost their row exclusivity in transduction mutants. GNAI3, another member of the mature row 1 complex, accumulated at mutant row 1 tips at considerably lower levels than in wild-type bundles. Alterations in stereocilia dimensions and in EPS8 distribution seen in transduction mutants were mimicked by block of transduction channels of cochlear explants in culture. In addition, proteins normally concentrated at mature row 2 tips were also distributed differently in transduction mutants; the heterodimeric capping protein subunit CAPZB and its partner TWF2 never concentrated at row 2 tips like they do in wild-type bundles. The altered distribution of marker proteins in transduction mutants was accompanied by increased variability in stereocilia length. Transduction channels thus specify and maintain row identity, control addition of new actin filaments to increase stereocilia diameter, and coordinate stereocilia height within rows.