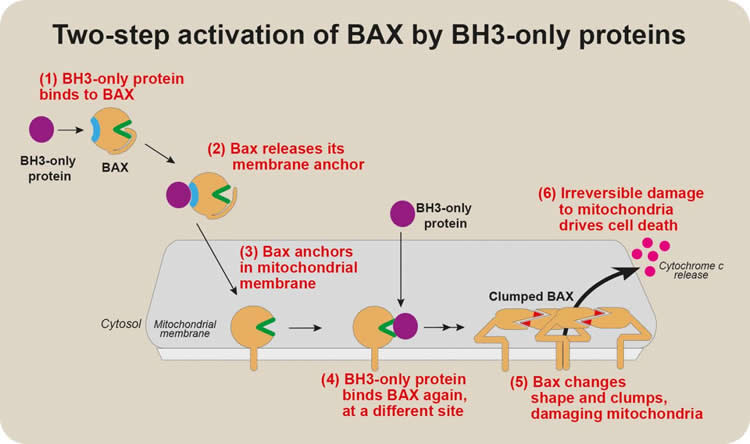
Summary: Two different parts to the BAX protein can bind to BH3-only, and these sites function at different stages of BAX activation. When BH3-only binds to one of the regions, BAX becomes able to damage mitochondria.
Source: Walter and Eliza Hall Institute
A ‘hit-and-run’ interaction between two proteins could be an important trigger for cell death, according to new research from Walter and Eliza Hall Institute researchers.
The researchers investigated the chain of events that activates the protein BAX, which is a crucial driver of apoptosis, the major form of cell death. Addressing a long-standing question in the field, they discovered that BAX is activated for cell death by transient interactions with so-called ‘BH3-only’ proteins at two distant sites on BAX.
The research, led by Dr Michael Dengler and Professor Jerry Adams, was published today in the journal Cell Reports.
At a glance
- Activation of the protein BAX is a key step in apoptosis, the programmed death of cells.
- Our researchers revealed a previously controversial ‘hit-and-run’ interaction between BAX and ‘BH3-only’ proteins that initiates the conversion of BAX into a lethal protein for cells.
- The discovery, which clarifies a long-standing question in apoptosis research, could underpin the development of new approaches to trigger or prevent the death of cells in certain diseases.
Triggering cell death
Apoptosis is the major way our bodies remove damaged or unwanted cells. Many different stimuli trigger apoptosis by turning on cell signalling pathways that activate BAX and its close relative BAK. Activated BAX and BAK create holes in the cell’s energy factories, the mitochondria. Once mitochondria are damaged, cells are compelled to die.
Professor Adams said a long-standing question in apoptosis research had been how BAX is triggered to move to mitochondria once cell death is triggered. “The events activating BAX once it has embedded on the surface membrane of mitochondria have been well-characterised – we know that death-inducing BH3-only proteins bind to BAX, changing its shape to damage the mitochondrial membrane,” he said.
“There had been hints that BH3-only proteins are also the signal for BAX to move from its location in a healthy cell’s cytosol – the liquid interior of the cell – to the mitochondria, but the experimental data supporting this were controversial and weak.”
Two-step activation
To understand how BAX interacts with BH3-only proteins, Dr Dengler and colleagues strategically altered different regions of BAX, subtly changing the protein’s structure. By comparing the behaviour of these mutant forms of BAX with that of normal, unmutated BAX, they could determine the function of different regions of BAX, he said.
“We discovered that two different parts of BAX could bind to BH3-only proteins,” he said. “Intriguingly, these sites functioned at different stages of BAX activation.”
“One site prompted BAX to move to the mitochondrial membrane. Binding of BH3-only proteins to this site on BAX changed BAX’s structure, releasing a ‘tail’ that anchors BAX to mitochondria. When BH3- only proteins bound the other site on BAX, BAX became able to damage the mitochondria.”
These two distinct steps in BAX activation had not previously been clearly distinguished.
“The first, early activation step had never been well characterised, because it appears to involve a transient ‘hit-and-run’ interaction between BAX and a BH3-only protein. We think this first step might be a way that BAX activation can be fine-tuned,” Dr Dengler said.
The research involved collaborations with structural biology and proteomics researchers at the Institute, aided by the Australian Synchrotron and the CSIRO Collaborative Crystallisation Centre. “Structural biology and proteomics were critical technologies for understanding how BAX is activated,” Dr Dengler said.
As well as explaining a key detail in how cell death is executed, the research may in the future lead to new classes of drugs that modify BAX function.
“BAX is a key mediator of cell death, and many major diseases involve either too little or too much cell death. Our discovery may eventually underpin the search for drugs that promote apoptosis by activating BAX, which may have potential for treating cancer. Conversely, drugs that block BAX activation could help to prevent the harmful cell death that occurs in neurodegenerative disorders or stroke,” Professor Adams said.
Funding: The research was supported by the Australian National Health and Medical Research Council, the USbased Leukemia and Lymphoma Society and the Victorian Government.
Source:
Walter and Eliza Hall Institute
Media Contacts:
Vanessa Solomon – Walter and Eliza Hall Institute
Image Source:
The image is credited to Walter and Eliza Hall Institute, Australia.
Original Research: Open access.
“BAX Activation: Mutations Near Its Proposed Non-canonical BH3 Binding Site Reveal Allosteric Changes Controlling Mitochondrial Association” Michael A. Dengler et al. Cell Reports. Published April 9 2019. doi:10.1016/j.celrep.2019.03.040
Abstract
BAX Activation: Mutations Near Its Proposed Non-canonical BH3 Binding Site Reveal Allosteric Changes Controlling Mitochondrial Association
Highlights
• BH3 binding to BAX groove essential for late but not early steps in BAX activation
• Mutations identified in BAX helices 1 and 6 near a proposed second BH3 binding site
• They reduced BH3 binding to this site and stabilized BAX in inactive conformations
• They revealed allosteric changes controlling BAX mitochondrial membrane association
Summary
To elicit apoptosis, BAX metamorphoses from an inert cytosolic monomer into homo-oligomers that permeabilize the mitochondrial outer membrane (MOM). A long-standing puzzle is that BH3 domains apparently activate BAX by not only its canonical groove but also a proposed site involving helices α1 and α6. Our mutagenesis studies reveal that late steps like oligomerization require activation through the groove but probably not earlier steps like MOM association. Conversely, α1 or α6 obstruction and alanine mutagenesis scanning implicate these helices early in BAX activation. The α1 and α6 mutations lowered BH3 binding, altered the BAX conformation, and reduced its MOM translocation and integration; their exposure of the BAX α1-α2 loop allosterically sequestered its α9 membrane anchor in the groove. The crystal structure of an α6 mutant revealed additional allosteric effects. The results suggest that the α1 and α6 region drives MOM association and integration, whereas groove binding favors subsequent steps toward oligomerization.