Summary: Researchers have identified a molecular process involved in gene expression in neurons which appears to play a key role in memory consolidation.
Source: UC Davis
The process by which memories are formed in the hippocampus region of the brain is complex. It relies on a precise choreography of interactions between neurons, neurotransmitters, receptors and enzymes.
A new mouse study led by researchers at the UC Davis School of Medicine has identified an intricate molecular process involving gene expression in the neurons that appears to play a critical role in memory consolidation. The research was published in Science Signaling.
“This is an exciting mechanism. It shows that an enzyme like phosphodiesterase is key in controlling gene expression necessary for memory consolidation,” said Yang K. Xiang, a professor in the Department of Pharmacology and senior author of the paper.
Xiang’s research focuses on understanding how dysregulation or impairment of cellular and molecular mechanisms in the heart and brain can lead to diseases like heart failure and Alzheimer’s.
Multiple steps in neuron appear critical for memory
The new study focuses on the central adrenergic system. The ability to pay attention, which is essential in learning and memory, is controlled by the central adrenergic system in the brain.
To understand the components critical for memory, the researchers looked at beta-2 adrenergic receptors. The receptors are present in different cell types throughout the body. They are also found on nerve cells in the hippocampal region of the brain.
The researchers show that when beta-2 adrenergic receptors are activated — through a series of molecular steps known as a signaling pathway — they stimulate the nucleus of the neuron to export an enzyme, phosphodiesterase 4D5 (PDE4D5).
Previous studies have identified PDE4D5 as having a role in promoting learning and memory.
Lack of phosphorylation leads to poor memory
A crucial step to stimulating this memory-related gene expression — the export of PDE4D5 — appears to be the attachment of a phosphate group (known as phosphorylation) to the receptor. This is accomplished by an enzyme known as a kinase.
The kinase involved in this case is a G-protein receptor kinase.
The researchers used genetically altered mice to test whether phosphorylation of the beta-2 adrenergic receptors by G-protein receptor kinase was necessary for gene expression — the export of the PDE4D5 enzyme.
The mice lacked a phosphorylation site on their beta-2 adrenergic receptors, meaning their neurons could not follow the normal signaling pathway when the receptors were activated.
The researchers found that, as expected, these genetically altered mice exhibited poor memory related to space and location. This is the same memory pathway that is disrupted during the early stages of Alzheimer’s disease.
However, when they provided the memory-impaired mice with a drug known as a PDE4 inhibitor (comparable to the PDE4D5 enzyme that would normally be exported), the mice’s ability to learn and retain memories was increased.
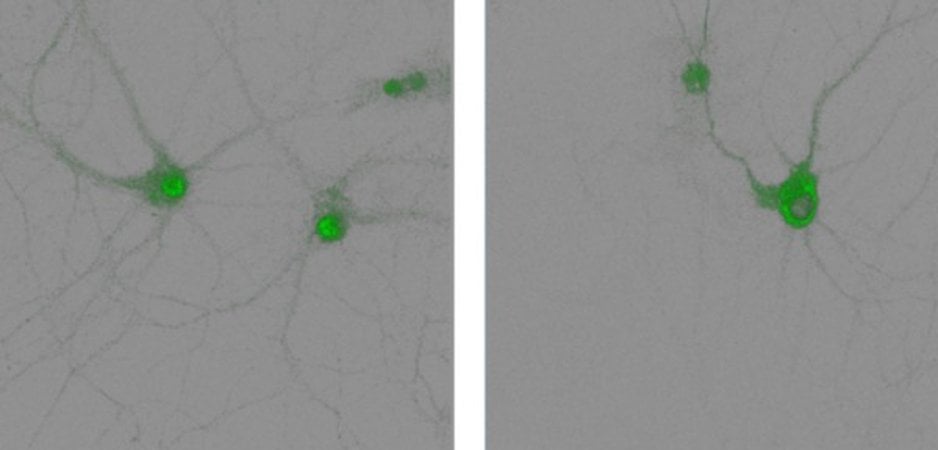
“The gene expression forms the material foundation of the memory in your brain. If you don’t have gene expression, you won’t have memory,” Xiang explained.
PDE inhibitors in Alzheimer’s have had mixed results
The use of PDE inhibitors is being explored for Alzheimer’s disease. Studies of the PDE5 inhibitor sildenafil, known as Viagra, have had mixed results. A 2021 NIH study found Viagra was associated with a reduced risk of Alzheimer’s disease, but a later study found Viagra was not associated with lower Alzheimer’s risk.
“We need to understand what is causing impairment in diseases like Alzheimer’s so we can find interventions that allow patients to regain ability or slow down the disease progression,” said Xiang. “This study highlights the potential of PDE inhibitors in rescuing memory in Alzheimer’s patients.”
Additional authors on the paper are Joseph M. Martinez, Aleksandra Jovanovic and Josephine de Chabot from UC Davis; Ao Shen from UC Davis and Guangzhou Medical University; Bing Xu from UC Davis and VA Northern California Health Care System; and Jin Zhang from, UC San Diego.
Funding: Funding for this research came from the National Institutes of Health (GM129376, T32GM099608, F99NS120523).
About this memory research news
Author: Lisa Howard
Source: UC Davis
Contact: Lisa Howard – UC Davis
Image: The image is in the public domain
Original Research: Open access.
“Arrestin-dependent nuclear export of phosphodiesterase 4D promotes GPCR-induced nuclear cAMP signaling required for learning and memory” by Yang K. Xiang et al. Science Signaling
Abstract
Arrestin-dependent nuclear export of phosphodiesterase 4D promotes GPCR-induced nuclear cAMP signaling required for learning and memory
G protein–coupled receptors (GPCRs) promote the expression of immediate early genes required for learning and memory.
Here, we showed that β2-adrenergic receptor (β2AR) stimulation induced the nuclear export of phosphodiesterase 4D5 (PDE4D5), an enzyme that degrades the second messenger cAMP, to enable memory consolidation.
We demonstrated that the endocytosis of β2AR phosphorylated by GPCR kinases (GRKs) mediated arrestin3-dependent nuclear export of PDE4D5, which was critical for promoting nuclear cAMP signaling and gene expression in hippocampal neurons for memory consolidation.
Inhibition of the arrestin3-PDE4D5 association prevented β2AR-induced nuclear cAMP signaling without affecting receptor endocytosis. Direct PDE4 inhibition rescued β2AR-induced nuclear cAMP signaling and ameliorated memory deficits in mice expressing a form of the β2AR that could not be phosphorylated by GRKs.
These data reveal how β2AR phosphorylated by endosomal GRK promotes the nuclear export of PDE4D5, leading to nuclear cAMP signaling, changes in gene expression, and memory consolidation.
This study also highlights the translocation of PDEs as a mechanism to promote cAMP signaling in specific subcellular locations downstream of GPCR activation.