Summary: Boosting levels of the DUSP4 protein could be a novel way of preventing and treating epilepsy.
Source: University of Illinois
Epileptic seizures often originate in small, localized areas of the brain where neurons abnormally fire in unison. These electrical impulses disrupt proper brain functioning and cause seizures. But what makes regions where seizures start different from parts of the brain where electrical impulses remain normal? More importantly, what prevents these epileptic centers from growing?
The answer to these questions may lie in a new discovery by researchers at the University of Illinois Chicago. Dr. Jeffrey Loeb and his colleagues found that a protein — called DUSP4 — was increased in healthy brain tissue directly adjacent to epileptic tissue. Their research suggests that boosting levels of DUSP4 could be a novel way of preventing or treating epilepsy.
Their findings are reported in the journal Neurobiology of Disease.
“If epileptic brain regions spread throughout the brain with nothing to stop them, the seizures would overwhelm the brain, it would not be survivable,” said Loeb, UIC professor and head of neurology and rehabilitation at the College of Medicine and corresponding author on the study. “We wondered if there were natural ways that epileptic brain areas are quarantined. We searched for genes at the border between epileptic and normal brain tissue that may help prevent the spread of epilepsy.”
Loeb and colleagues analyzed thousands of genes in tissues from 20 patients who underwent surgery to treat their epilepsy. During these surgical procedures, brain tissue from epileptic areas and directly adjacent, non-epileptic tissue was removed. Tissue not required for pathological evaluation was stored in the University of Illinois NeuroRepository — a human brain tissue bank and research database that links clinical, radiological, physiological, histological and molecular/genomic data to thousands of human tissue samples.
The researchers used a mathematical modeling technique called cluster analysis to sort through huge numbers of genes from the epileptic versus the nonepileptic tissue. They identified a number of genes that were increased — or “upregulated” — in or near epileptic tissues and the observed that DUSP4 fell into a different cluster than most of the pro-epileptic genes.
In previous research, Loeb and colleagues identified a signaling pathway that was highly upregulated in areas of the brain where epileptic seizures started. In an animal model, suppression of the pathway –known as the mitogen-activated protein kinase, or MAPK, pathway — reduced epileptic electrical activity in the brain.
“We were excited about DUSP4 because it is known to be a potent MAPK pathway inhibitor in cancer cells,” Loeb said. “Seeing this gene activated at the borders and shutting off MAPK signaling genes in the human brain led us to believe that the protein cordons off epileptic regions so that they don’t enlarge or spread, similar to how in a ship you might get a leak in one area, but you can close and seal off doors to keep the leak isolated. That’s how we think DUSP4 is working to keep epileptic focal points from enlarging.”
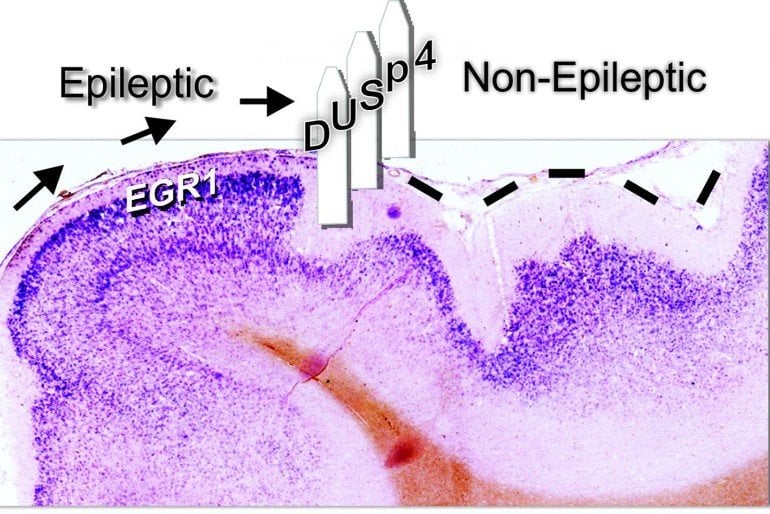
In addition to the gene, when the researchers went back to look at the protein’s levels in their tissue samples, they found that tissue from brain regions with lower epileptic activity had lower MAPK activity and higher levels of the DUSP4 protein.
Loeb and colleagues currently are investigating potential drugs that can upregulate or augment the activity of DUSP4 to help treat or even prevent epilepsy.
“These DUSP4-targeting drugs would represent a new kind of ‘disease-modifying’ treatment for epilepsy, which currently does not exist,” Loeb said.
Allison Kirchner and Fabien Dachet of UIC and Shruti Bagla of Wayne State University are co-authors on the paper.
Funding: This work was supported by grants from the National Institutes of Health (NS109515, NS083527) and an American Epilepsy Society Predoctoral Fellowship.
About this Epilepsy research news
Source: University of Illinois
Contact: Jackie Carey – University of Illinois
Image: Image is credited to UIC/Jeffrey Loeb.
Original Research: Open access.
“DUSP4 appears to be a highly localized endogenous inhibitor of epileptic signaling in human neocortex” by Jeffrey Loeb et al. Neurobiology of Disease.
Abstract
DUSP4 appears to be a highly localized endogenous inhibitor of epileptic signaling in human neocortex
Background
We previously identified the Mitogen Activated Protein Kinase (MAPK) pathway as focally upregulated in brain regions with high epileptic activity and showed that inhibition of MAPK signaling reduces epileptic spiking in an animal model. Here we examined how activators and inhibitors of the MAPK pathway are expressed in human epileptic cortex and how these could contribute to the localization of epileptic signaling.
Methods
We localized gene and protein expression in human epileptic neocortical tissues based on epileptic activities from 20 patients based on long-term intracranial recordings. Follow-up mechanistic studies by depolarization of human Sh-SY5Y cell line were used to model epileptic activity in the human brain.
Results
A clustering algorithm of differentially expressed genes identified a unique gene expression cluster distinct from other MAPK genes. Within this cluster was dual specificity phosphatase 4 (DUSP4), a potent MAPK inhibitor. In situ hybridization studies revealed focal patches of DUSP4 mRNA in layer 2/3 brain regions associated with a dramatic reduction in MAPK signaling genes. In vitro depolarization led to the rapid and transient induction of DUSP4 protein, which, in turn, reduced MAPK activity. Activity-dependent induction of DUSP4 protein was transient and required MAPK signaling. Human epileptic brain regions with lower epileptic activity had lower MAPK activity and higher DUSP4 protein levels.
Discussion
DUSP4 is a highly localized, endogenous feedback inhibitor of pro-epileptogenic MAPK signaling in the human epileptic brain. Increasing DUSP4 expression could therefore be a novel therapeutic approach to prevent the development and spread of epileptic circuits.
Significance statement
Epilepsy is a chronic debilitating disease. Once it develops, epileptic circuits often persist throughout life. Fortunately, in focal forms of epilepsy, these circuits can remain highly localized and are amenable to surgical resections, suggesting that endogenous mechanisms restrict their spread to other brain regions. Using a high-throughput genomic analysis of human epileptic brain regions, we identified DUSP4 as an activity-dependent inhibitor of MAPK signaling expressed in focal patches surrounding human neocortical epileptic brain regions. Our results suggest that DUSP4, through local inhibition of MAPK signaling, acts as an endogenous, spatially segregated safety mechanism to prevent the spread of epileptic activity. Augmenting DUSP4 expression could be a novel disease-modifying approach to prevent or treat human epilepsy.






