Summary: Researchers have decoded the 3-D architecture of the main SARS-CoV-2 protease. The protein is involved in the reproduction of the virus. The findings may help in the systematic development of drugs to inhibit the reproduction of the virus, potentially lowering COVID-19 infections.
Source: Helmholtz
A coronavirus is keeping the world in suspense. SARS-CoV-2 is highly infectious and can cause severe pneumonia with respiratory distress (COVID-19). Scientists are doing research in order to prevent the viruses from multiplying. A team from the University of Lübeck has now found a promising approach. Using the high-intensity X-ray light from the Berlin synchrotron source BESSY II, they have decoded the three-dimensional architecture of the main protease of SARS-CoV-2. This protein is involved in the reproduction of the virus. Analysing its 3D architecture allows the systematic development of drugs which inhibit the reproduction of the virus.
Teams around the world are working hard to develop active substances against SARS-CoV-2. The structural analysis of functional proteins of the virus is very helpful for this goal. The function of a protein is closely related to its 3-D architecture. If this 3-D architecture is known, it is possible to identify specific points of attack for active substances.
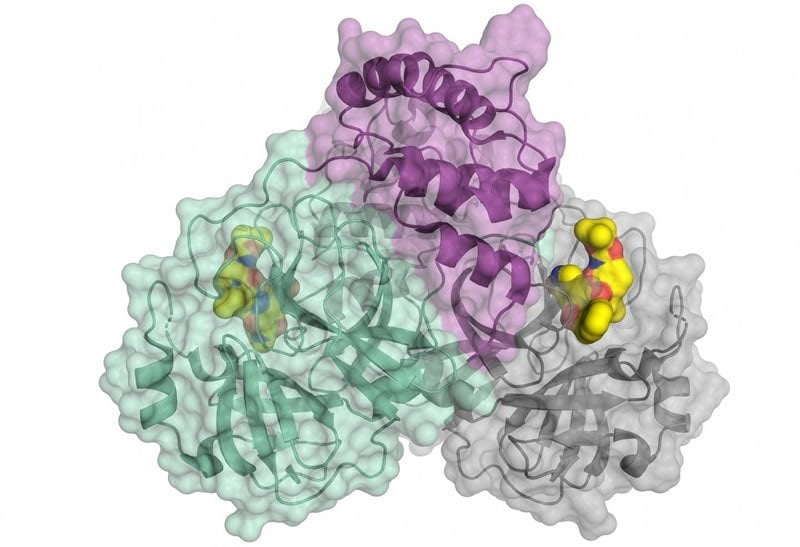
A special protein is responsible for the reproduction of the viruses: the viral main protease (Mpro or also 3CLpro). A team led by Prof. Dr. Rolf Hilgenfeld, University of Lübeck, has now decoded the 3-D architecture of the main protease of SARS-CoV-2. The researchers have used the high-intensity X-ray light from the BESSY II facility of the Helmholtz-Zentrum Berlin.
“For such issues of highest relevance, we can offer fast track access to our instruments,” says Dr. Manfred Weiss, who heads the Research Group Macromolecular Crystallography (MX) at HZB. At the so-called MX instruments tiny protein crystals can be analysed with highly brilliant X-ray light. The images contain information about the 3-D architecture of the protein molecules. The complex shape of the protein molecule and its electron density is then calculated by computer algorithms.
The 3-D architecture provides concrete starting points for developing active substances or inhibitors. These drugs could dock specifically to target points of the macromolecule and impede its function. Rolf Hilgenfeld is a world-renowned expert in the field of virology and already developed an inhibitor against the SARS-virus during the 2002/2003 SARS pandemic. In 2016, he succeeded in deciphering an enzyme of the Zika virus.
Source:
Helmholtz
Media Contacts:
Press Office – Helmholtz
Image Source:
The image is credited to HZB.
Original Research: Open access
“Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors”. Linlin Zhang et al.
Science doi:10.1126/science.abb3405.
Abstract
Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors
The COVID-19 pandemic caused by SARS-CoV-2 is a global health emergency. An attractive drug target among coronaviruses is the main protease (Mpro, 3CLpro), due to its essential role in processing the polyproteins that are translated from the viral RNA. We report the X-ray structures of the unliganded SARS-CoV-2 Mpro and its complex with an α-ketoamide inhibitor. This was derived from a previously designed inhibitor but with the P3-P2 amide bond incorporated into a pyridone ring to enhance the half-life of the compound in plasma. Based on the structure, we developed the lead compound into a potent inhibitor of the SARS-CoV-2 Mpro. The pharmacokinetic characterization of the optimized inhibitor reveals a pronounced lung tropism and suitability for administration by the inhalative route.