Summary: Brown adipose fat (BAT) may help to protect against diabetes and obesity. BAT serves as a key metabolic filter that controls branched-chain amino acid clearance, contributing to better overall metabolic health.
Source: Rutgers University
Rutgers and other scientists have discovered how brown fat, also known as brown adipose tissue, may help protect against obesity and diabetes. Their study in the journal Nature adds to our knowledge about the role of brown fat in human health and could lead to new medications for treating obesity and type 2 diabetes.
Brown fat is considered a heat organ. People have a few grams of it in areas including the neck, collarbone, kidneys and spinal cord. When activated by cool temperatures, brown fat uses sugar and fat from the blood to generate heat in the body.
The study found that brown fat could also help the body filter and remove branched-chain amino acids (BCAAs) from the blood. BCAAs (leucine, isoleucine and valine) are found in foods like eggs, meat, fish, chicken and milk, but also in supplements used by some athletes and people who want to build muscle mass.
In normal concentrations in the blood, these amino acids are essential for good health. In excessive amounts, they’re linked to diabetes and obesity. The researchers found that people with little or no brown fat have reduced ability to clear BCAAs from their blood, and that may lead to the development of obesity and diabetes.
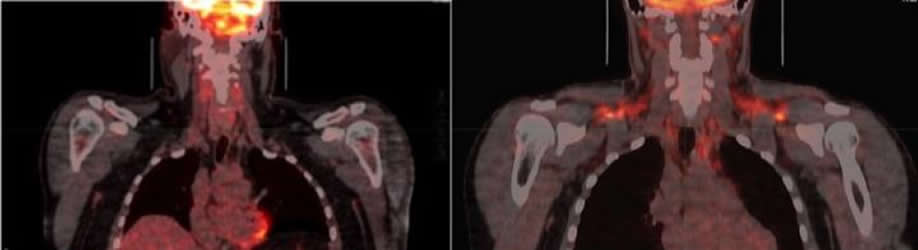
The study also solved a 20-plus year mystery about brown fat: how BCAAs enter the mitochondria that generate energy and heat in cells. The scientists discovered that a novel protein (called SLC25A44) controls the rate at which brown fat clears the amino acids from the blood and uses them to produce energy and heat.
“Our study explains the paradox that BCAA supplements can potentially benefit those with active brown fat, such as healthy people, but can be detrimental to others, including the elderly, obese and people with diabetes,” said co-author Labros S. Sidossis, a Distinguished Professor who chairs the Department of Kinesiology and Health in the School of Arts and Sciences at Rutgers University-New Brunswick. He is also a professor in the Department of Medicine at Rutgers Robert Wood Johnson Medical School in Rutgers Biomedical and Health Sciences.
Researchers next need to determine whether uptake of BCAAs by brown fat can be controlled by environmental factors – such as exposure to mildly cold temperatures (65 degrees Fahrenheit) or consumption of spicy foods – or by drugs. This could improve blood sugar levels that are linked to diabetes and obesity, Sidossis said.
Source:
Rutgers University
Media Contacts:
Todd Bates – Rutgers University
Image Source:
The image is credited to Labros Sidossis/Rutgers University.
Original Research: Closed access
“BCAA catabolism in brown fat controls energy homeostasis through SLC25A44”. Takeshi Yoneshiro, Qiang Wang, Kazuki Tajima, Mami Matsushita, Hiroko Maki, Kaori Igarashi, Zhipeng Dai, Phillip J. White, Robert W. McGarrah, Olga R. Ilkayeva, Yann Deleye, Yasuo Oguri, Mito Kuroda, Kenji Ikeda, Huixia Li, Ayano Ueno, Maki Ohishi, Takamasa Ishikawa, Kyeongkyu Kim, Yong Chen, Carlos Henrique Sponton, Rachana N. Pradhan, Homa Majd, Vanille Juliette Greiner, Momoko Yoneshiro, Zachary Brown, Maria Chondronikola, Haruya Takahashi, Tsuyoshi Goto, Teruo Kawada, Labros Sidossis, Francis C. Szoka, Michael T. McManus, Masayuki Saito, Tomoyoshi Soga & Shingo Kajimura.
Nature. doi:10.1038/s41586-019-1503-x
Abstract
BCAA catabolism in brown fat controls energy homeostasis through SLC25A44
Branched-chain amino acid (BCAA; valine, leucine and isoleucine) supplementation is often beneficial to energy expenditure; however, increased circulating levels of BCAA are linked to obesity and diabetes. The mechanisms of this paradox remain unclear. Here we report that, on cold exposure, brown adipose tissue (BAT) actively utilizes BCAA in the mitochondria for thermogenesis and promotes systemic BCAA clearance in mice and humans. In turn, a BAT-specific defect in BCAA catabolism attenuates systemic BCAA clearance, BAT fuel oxidation and thermogenesis, leading to diet-induced obesity and glucose intolerance. Mechanistically, active BCAA catabolism in BAT is mediated by SLC25A44, which transports BCAAs into mitochondria. Our results suggest that BAT serves as a key metabolic filter that controls BCAA clearance via SLC25A44, thereby contributing to the improvement of metabolic health.






