Summary: Cryo-electron microscopy captures detailed snapshots of the GABAB receptor protein contorts as it interacts with GABA.
Source: USC
As the body goes about its daily business, molecules called neurotransmitters control the level of electrical activity within the brain. Interacting with protein receptors nestled in the membrane that makes up the outer border of a neuron, neurotransmitters open and close portals that control the flow of ions in and out of the cell.
Gamma-aminobutyric acid, or GABA, dominates as one of the most important inhibitory neurotransmitters in the brain. Its primary role is to calm brain activity, lowering the number of signals firing in the brain in balance with the activity of other neurotransmitters that ramp up brain activity. (It’s even sold as a nutritional supplement to promote calm and improve sleep.)
When GABA is unable to inhibit brain signaling, the imbalance in activity can lead to a number of health issues including anxiety or mood disorders, increased pain, muscle spasms and, in extreme cases, even epilepsy.
In a study published in Nature on June 17, scientists at USC Dornsife College of Letters, Arts and Sciences and the Bridge Institute at the USC Michelson Center for Convergent Bioscience figured out how GABA interacts with a key protein receptor called GABAB.
The study, done in collaboration with researchers at Stanford University, the Stanford Linear Accelerator Center and the Université de Montpellier in France, paints a clear picture of how GABA changes the shape of GABAB and reveal a clear target for new drugs.
Ultra-high definition views of a shape-shifter
The researchers used cryo-electron microscopy, or cryo-EM, to capture never-before-seen detailed snapshots of the GABAB receptor protein twisting and contorting as it interacts with GABA.
The GABAB receptor protein rests across the cellular membrane of neurons and is composed of two subunits with similar shapes: GB1, which recognizes GABA, and GB2, which relays the signal from GB1 into the cell’s interior.
The cryo-EM images provide blueprints of how, as GABA interacts with GB1, a ripple effect moves through the entire protein until a cavity opens in the part of GB2 that faces inside the cell. Once revealed, this cavity in GB2 can interact with and activate proteins within the cell that control the neuron’s activity.
The three-dimensional structural images offer promise for improved therapies for neurological disorders, according to Vadim Cherezov, professor of chemistry at USC Dornsife and a corresponding author on the study. “These blueprints can be used to design new therapeutic drugs that could affect different conformational states and thus act more precisely.”
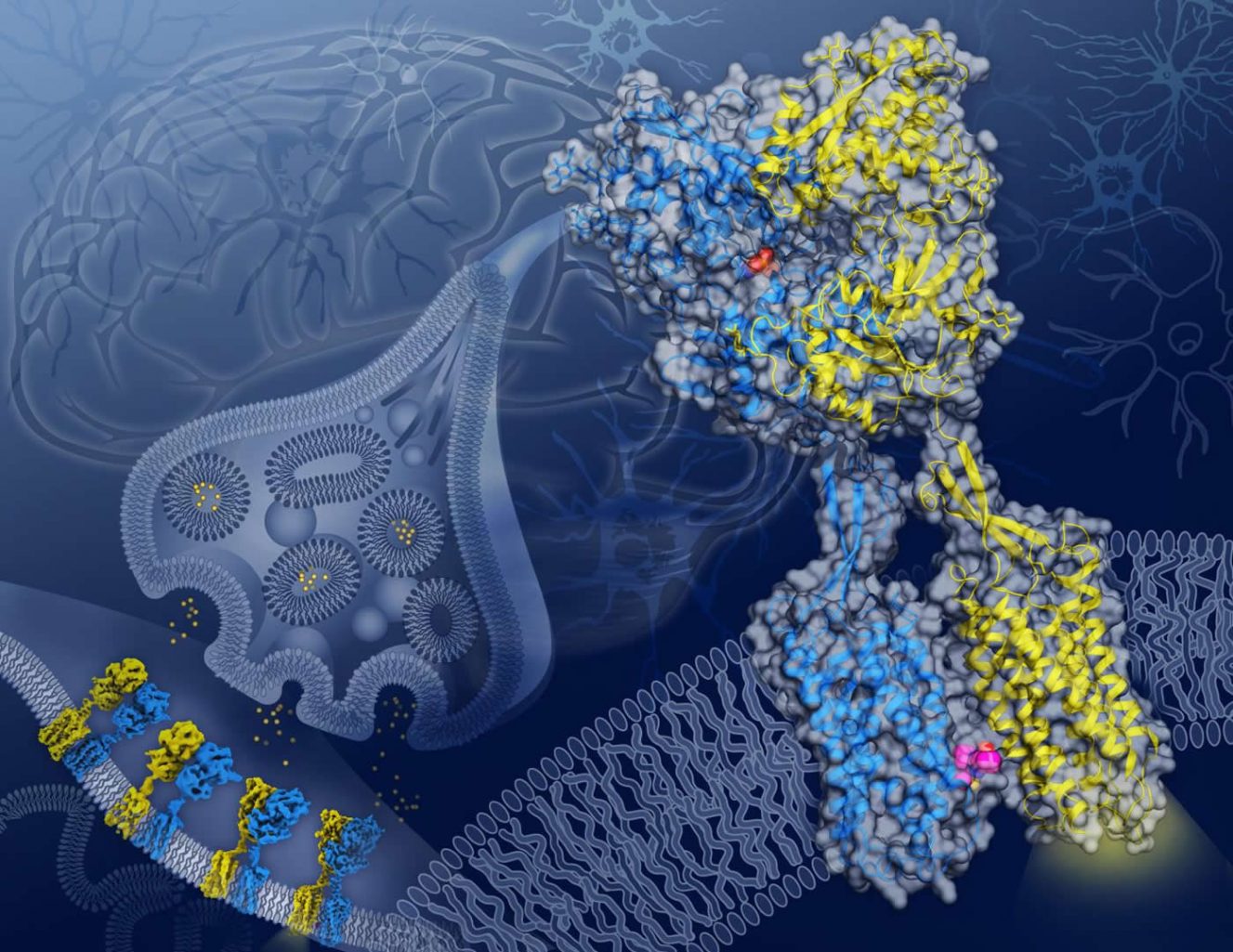
Equally important if not more so, the images also reveal a critical location on the GABAB receptor protein, where GB1 and GB2 meet within the cell membrane when the receptor is activated. Previously unknown, this GB1-GB2 interface is a prime target for a type of drug called a PAM (short for positive allosteric modulator).
PAMs offer promise for the development of new therapeutic drugs because they don’t replace the action of naturally occurring molecules such as GABA but rather fine-tune how the receptor behaves, leading to fewer side-effects, Cherezov said.
“There are many therapeutic areas in which targeting the GABAB receptor protein could be beneficial; however, such direct interventions may come with significant side-effects,” he said. “Developments of PAMs facilitated by our structure images could lead to a new generation of safer drugs targeting this receptor.”
Authors on the study include USC Dornsife’s Hamidreza Shaye, Andrii Ishchenko, Jordy Homing Lam, Gye Won Han, and Vsevolod Katritch; Cornelius Gati of Stanford University and SLAC; and Li Xue, Philippe Rondard and Jean-Philippe Pin of Université de Montpellier.
Funding: The study was funded by National Institutes of Health grant R35 GM127086 (Cherezov); the Department of Energy, Laboratory Directed Research and Development program at SLAC National Accelerator Laboratory, under contract DE-AC02-76SF00515; and by the Fondation pour la Recherche Médicale award DEQ20170336747 (Pin).
About this neuroscience research article
Source:
USC
Media Contacts:
Jim Key – USC
Image Source:
The image is credited to Yekaterina Kadyshevskaya/Bridge Institute at the USC Michelson Center for Convergent Bioscience.
Original Research: Closed access
“Structural basis of the activation of a metabotropic GABA receptor”. by Hamidreza Shaye, Andrii Ishchenko, Jordy Homing Lam, Gye Won Han, Li Xue, Philippe Rondard, Jean-Philippe Pin, Vsevolod Katritch, Cornelius Gati & Vadim Cherezov.
Nature doi:10.1038/s41586-020-2408-4
Abstract
Structural basis of the activation of a metabotropic GABA receptor
Metabotropic γ-aminobutyric acid receptors (GABAB) are involved in the modulation of synaptic responses in the central nervous system and have been implicated in neuropsychological conditions that range from addiction to psychosis. GABAB belongs to class C of the G-protein-coupled receptors, and its functional entity comprises an obligate heterodimer that is composed of the GB1 and GB2 subunits. Each subunit possesses an extracellular Venus flytrap domain, which is connected to a canonical seven-transmembrane domain. Here we present four cryo-electron microscopy structures of the human full-length GB1–GB2 heterodimer: one structure of its inactive apo state, two intermediate agonist-bound forms and an active form in which the heterodimer is bound to an agonist and a positive allosteric modulator. The structures reveal substantial differences, which shed light on the complex motions that underlie the unique activation mechanism of GABAB. Our results show that agonist binding leads to the closure of the Venus flytrap domain of GB1, triggering a series of transitions, first rearranging and bringing the two transmembrane domains into close contact along transmembrane helix 6 and ultimately inducing conformational rearrangements in the GB2 transmembrane domain via a lever-like mechanism to initiate downstream signalling. This active state is stabilized by a positive allosteric modulator binding at the transmembrane dimerization interface.






