Enzymes rarely have one job. So, attempts to shut down the enzyme that causes the hallmarks of Alzheimer’s disease often mean side effects, because these therapies prevent the enzyme from carrying out many other functions. A study appearing February 25 in Cell Reports presents a new therapeutic strategy: blocking the most harmful behavior enzyme while allowing it to work normally otherwise. This potential approach now needs to be further developed and tested in pre-clinical trials.
In the brains of patients with Alzheimer’s disease (AD), amyloid precursor protein is broken apart, and the resulting fragments–β-amyloid peptides, or Aβ peptides–aggregate to form plaques. Aβ peptides are produced by the action of two enzymes called beta- and gamma-secretases. Inhibiting either of these enzymes would block the production of toxic Aβ peptides; however, attempts to inhibit gamma-secretase caused problems in clinical trials because the enzyme also cleaves more than 20 other proteins important for normal physiology. β-secretase is now considered an alternative therapeutic target for AD, and a wide variety of inhibitors have been developed; however, β-secretase also cleaves several other proteins with normal functions in the body.
In their latest research, Lawrence Rajendran, of the University of Zurich in Switzerland, and his colleagues discovered that, unlike non-amyloid proteins, the Alzheimer’s-associated amyloid precursor protein is cleaved by β-secretase in membrane-bound compartments inside cells, called endosomes. Exploiting this compartmentalization, the team developed an endosomally-targeted β-secretase inhibitor that specifically blocked cleavage of amyloid precursor protein but not non-amyloid proteins. This is the first time such specificity has been achieved, and it thus provides a potentially promising way to treat AD without causing major side effects.
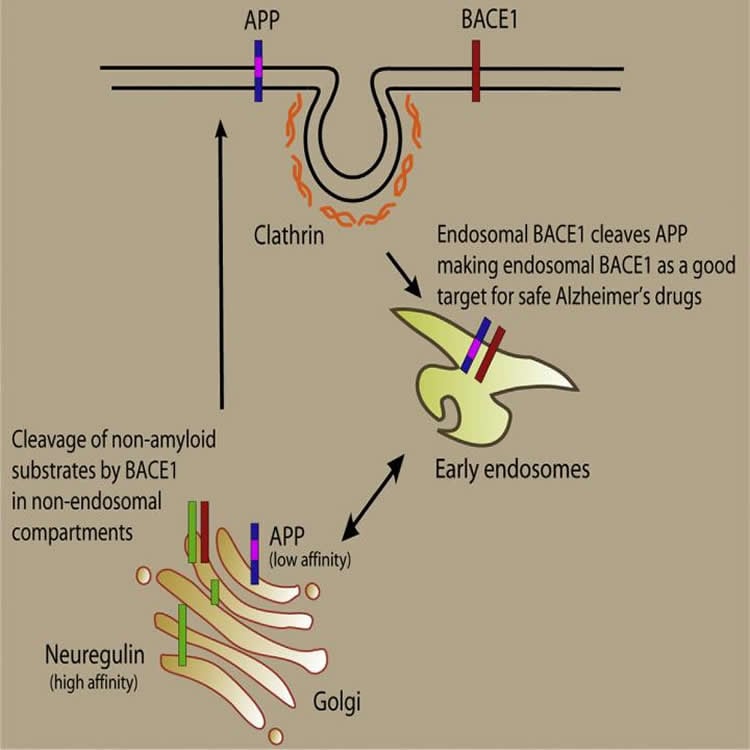
“The current β-secretase inhibitors inhibit both the Alzheimer’s disease process and physiologically relevant processes, and this would be a major problem, similar to the gamma-secretase inhibitors that failed in the clinic; however, with our strategy, we now can specifically inhibit the Alzheimer’s process thereby avoiding any side effects,” says Rajendran. He and his team plan to develop this inhibitor further and test it in clinical trials.
Funding: The authors acknowledge financial support from the Swiss National Science Foundation, the Velux Foundation, the Cure Alzheimer Fund, the Baugarten Stiftung, and the German Federal Ministry for Education and Research.
Source: Joseph Caputo – Cell Press
Image Credit: Image is credited to Ben Halima et al./Cell Reports 2016.
Original Research: Full open access research for “Specific Inhibition of β-Secretase Processing of the Alzheimer Disease Amyloid Precursor Protein” by Saoussen Ben Halima, Sabyashachi Mishra, K. Muruga Poopathi Raja, Michael Willem, Antonio Baici, Kai Simons, Oliver Brüstle, Philipp Koch, Christian Haass, Amedeo Caflisch, and Lawrence Rajendran in Cell Reports. Published online February 25 2016 doi:10.1016/j.celrep.2016.01.076
Abstract
Specific Inhibition of β-Secretase Processing of the Alzheimer Disease Amyloid Precursor Protein
Highlights
•The AD-linked protease BACE1 cleaves APP to produce toxic β-amyloid peptides
•BACE1 also cleaves the non-amyloid substrates NRG1 and L1
•BACE1 cleavage of NRG1 and L1 is endocytosis-independent, unlike the cleavage of APP
•The endosomally targeted BACE1 inhibitor spares NRG1 and L1 but inhibits APP processing
Summary
Development of disease-modifying therapeutics is urgently needed for treating Alzheimer disease (AD). AD is characterized by toxic β-amyloid (Aβ) peptides produced by β- and γ-secretase-mediated cleavage of the amyloid precursor protein (APP). β-secretase inhibitors reduce Aβ levels, but mechanism-based side effects arise because they also inhibit β-cleavage of non-amyloid substrates like Neuregulin. We report that β-secretase has a higher affinity for Neuregulin than it does for APP. Kinetic studies demonstrate that the affinities and catalytic efficiencies of β-secretase are higher toward non-amyloid substrates than toward APP. We show that non-amyloid substrates are processed by β-secretase in an endocytosis-independent manner. Exploiting this compartmentalization of substrates, we specifically target the endosomal β-secretase by an endosomally targeted β-secretase inhibitor, which blocked cleavage of APP but not non-amyloid substrates in many cell systems, including induced pluripotent stem cell (iPSC)-derived neurons. β-secretase inhibitors can be designed to specifically inhibit the Alzheimer process, enhancing their potential as AD therapeutics without undesired side effects.
“Specific Inhibition of β-Secretase Processing of the Alzheimer Disease Amyloid Precursor Protein” by Saoussen Ben Halima, Sabyashachi Mishra, K. Muruga Poopathi Raja, Michael Willem, Antonio Baici, Kai Simons, Oliver Brüstle, Philipp Koch, Christian Haass, Amedeo Caflisch, and Lawrence Rajendran in Cell Reports. Published online February 25 2016 doi:10.1016/j.celrep.2016.01.076







