Summary: A new study argues against the rejuvenating properties of young blood.
Source: UC Berkeley.
A new study from UC Berkeley found that tissue health and repair dramatically decline in young mice when half of their blood is replaced with blood from old mice. The study argues against the rejuvenating properties of young blood and points to old blood, or molecules within, as driving the aging process.
“Our study suggests that young blood by itself will not work as effective medicine,” said Irina Conboy, associate professor in the Department of Bioengineering at UC Berkeley. “It’s more accurate to say that there are inhibitors in old blood that we need to target to reverse aging.”
The study was published today in the journal Nature Communications. The research was supported by funding from the National Institutes of Health, SENS Research Foundation, Rogers’ Family and Calico.
In 2005, Conboy and colleagues published a study in Nature that found evidence for tissue rejuvenation in older mice when they are surgically joined to younger mice so that blood is exchanged between the two. Despite remaining questions about the mechanism underlying this rejuvenation, media coverage of the study fixated on the potential of young blood to reverse the aging process, and on comparisons to vampires, which was not the takeaway from the study, Conboy said. In the years since the 2005 study, scientists have spent millions to investigate the potential medical properties of youthful blood with enterprises emerging to infuse old people with young blood.
“What we showed in 2005 was evidence that aging is reversible and is not set in stone,” Conboy said. “Under no circumstances were we saying that infusions of young blood into elderly is medicine.”
Blood exchange in humans is FDA-approved for a few devastating illnesses (auto-immunity, for example, where self-reacting antibodies are removed), but high volume or repeated additions of blood or its components to genetically different people is known to have side effects of immune rejection, leading to organ failure.
While the experimental model used in the 2005 study found evidence that some aspects of aging may be reversed, the techniques used in the study do not allow scientists to precisely control the exchange of blood, which is necessary to dig deeper into blood’s effect on aging.
When two mice are sutured together, a technique called parabiosis, blood is not the only thing that is exchanged in this setup; organs are also shared, so old mice get access to younger lungs, thymus-immune system, heart, liver and kidneys. In surgical suturing it takes weeks to a month for the effects of blood to take place and the precise timing is not actually known. Nor is the precise amount of the exchanged blood.
In the new study, Conboy and colleagues developed an experimental technique to exchange blood between mice without joining them so that scientists can control blood circulation and conduct precise measurements on how old mice respond to young blood, and vice versa. In the new system, mice are connected and disconnected at will, removing the influence of shared organs or of any adaptation to being joined. One of the more surprising discoveries of this study was the very quick onset of the effects of blood on the health and repair of multiple tissues, including muscle, liver and brain. The effects were seen around 24 hours after exchange.
With the new experimental setup, the research team repeated the experiments from 2005. In each test, blood was exchanged between an old mouse and a young mouse until each mouse had half its blood from the other. The researchers then tested various indicators of aging in each mouse, such as liver cell growth as well as liver fibrosis and adiposity (fat), brain cell development in the region that is needed for learning and memory, muscle strength and muscle tissue repair. In many of these experiments, older mice that received younger blood saw either slight or no significant improvements compared to old mice with old blood. Young mice that received older blood, however, saw large declines in most of these tissues or organs.
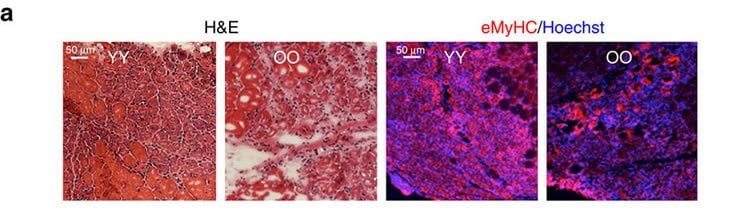
The most telling data was found when researchers tested blood’s impact on new neuron production in the area of the brain where memory and learning are formed. In these experiments, older mice showed no significant improvement in brain neuron stem cells after receiving younger blood, but younger mice that received older blood saw a more than twofold drop in brain cell development compared to normal young mice. The researchers think that many benefits seen in old mice after receiving young blood might be due to the young blood diluting the concentration of inhibitors in the old blood.
“Under no circumstances did young blood improve brain neurogenesis in our experiments,” Conboy said. “Old blood appears to have inhibitors of brain cell health and growth, which we need to identify and remove if we want to improve memory.”
The research team has begun to investigate specific molecules in old blood that might cause inhibition of cell development, but future experiments are needed for a clear picture of why young animals are worse off with old blood.
Funding: The research was supported by funding from the National Institutes of Health, SENS Research Foundation, Rogers’ Family and Calico.
Source: Brett Israel – UC Berkeley
Image Source: NeuroscienceNews.com image is credited to Conboy et al./Nature Communications.
Original Research: Full open access research for “A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood” by Justin Rebo, Melod Mehdipour, Ranveer Gathwala, Keith Causey, Yan Liu, Michael J. Conboy & Irina M. Conboy in Nature Communications. Published online November 22 2016 doi:10.1038/ncomms13363
[cbtabs][cbtab title=”MLA”]UC Berkeley “Young Blood Does Not Reverse Aging in Old Mice.” NeuroscienceNews. NeuroscienceNews, 22 November 2016.
<https://neurosciencenews.com/aging-blood-neuroscience-5576/>.[/cbtab][cbtab title=”APA”]UC Berkeley (2016, November 22). Young Blood Does Not Reverse Aging in Old Mice. NeuroscienceNew. Retrieved November 22, 2016 from https://neurosciencenews.com/aging-blood-neuroscience-5576/[/cbtab][cbtab title=”Chicago”]UC Berkeley “Young Blood Does Not Reverse Aging in Old Mice.” https://neurosciencenews.com/aging-blood-neuroscience-5576/ (accessed November 22, 2016).[/cbtab][/cbtabs]
Abstract
A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood
Heterochronic parabiosis rejuvenates the performance of old tissue stem cells at some expense to the young, but whether this is through shared circulation or shared organs is unclear. Here we show that heterochronic blood exchange between young and old mice without sharing other organs, affects tissues within a few days, and leads to different outcomes than heterochronic parabiosis. Investigating muscle, liver and brain hippocampus, in the presence or absence of muscle injury, we find that, in many cases, the inhibitory effects of old blood are more pronounced than the benefits of young, and that peripheral tissue injury compounds the negative effects. We also explore mechanistic explanations, including the role of B2M and TGF-beta. We conclude that, compared with heterochronic parabiosis, heterochronic blood exchange in small animals is less invasive and enables better-controlled studies with more immediate translation to therapies for humans.
“A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood” by Justin Rebo, Melod Mehdipour, Ranveer Gathwala, Keith Causey, Yan Liu, Michael J. Conboy & Irina M. Conboy in Nature Communications. Published online November 22 2016 doi:10.1038/ncomms13363