Summary: A new study reports the retinas from our earliest vertebrate ancestors had cone like receptors, allowing them to see both in daylight and at night.
Source: NIH/National Eye Institute.
Genesis of our rod-dominant retina may explain ancestors’ survival through ‘nocturnal bottleneck’.
Retinas from our earliest vertebrate ancestors had cone-like photoreceptors, presumably allowing them to see in daylight, but little ability to see at night. Then, millions of years ago in the Mesozoic era, and in relatively short order, mammals emerged that had retinas with predominantly rod photoreceptors, allowing for them to see at night perhaps to hunt for food while their dinosaur predators were dozing. Now a new study led by researchers the National Eye Institute suggests how the genesis of rod photoreceptors may have occurred to give rise to nocturnal mammals.
The findings address a key piece of the evolution puzzle: How did early mammals so quickly evolve to have highly sensitive night vision? They also suggest how mammals evolved past the “nocturnal bottleneck”, a theory that attempts to explain why most mammals today are either nocturnal or at least able to see in dim light. As it turns out, seeing well at night not only enabled our ancestors to survive, but it allowed them to thrive, so much so that their retinal traits were well preserved and passed on through millions of years of evolution.
The study findings address another mystery: Why are rods the dominant photoreceptors in our retinas? “Despite the fact that sharp, acute vision would seem to be more important to our diurnal lifestyle, our retinas are predominantly made up of rods; only 5 percent of the retina’s photoreceptors are cones,” said the collaborative study’s lead investigator, Anand Swaroop, Ph.D., chief of NEI’s Neurobiology-Neurodegeneration and Repair Laboratory. “The simplest explanation that we have is that this excess of rods in the majority of mammals were recruited from cones to overcome this nocturnal bottleneck.”
By studying mice, Swaroop and his team observed that a certain type of cone cell could be coaxed into becoming a rod photoreceptor within days after birth. They hypothesized that this transformation of a cone into a rod cell was regulated by a protein called Nrl, which binds to DNA and directs a retinal cell that is fated to become a cone to become a rod instead. The team had long observed that when mice are bred to have loss of Nrl, they fail to develop rods in their retina.
In the current study, they tested their theory by isolating rod cells from normal wild-type mice and sequencing their expressed genes. Genetically, the rod cells contained evidence of cone gene expression, but the expression of these cone genes diminishes once the rod cell matures. “This expression of cone cell genes is a kind of footprint indicating that the rod cells may have originated from cone cells,” Swaroop explained. Studies of mouse retina during key stages of development following birth supported their theory that early in development cone genes are expressed, but this expression is turned off once Nrl redirects the cell to become a rod, and the rod matures.
Additional evidence was generated by tracing the lineage of rod and cone cells in the mouse retina. Transgenic mouse lines were bred that allowed the researchers to permanently tag cone-related proteins in the retina of their offspring. Those cells could then be traced from birth, allowing the researchers to confirm that the majority of rods in mice have cone-cell activity during their development.
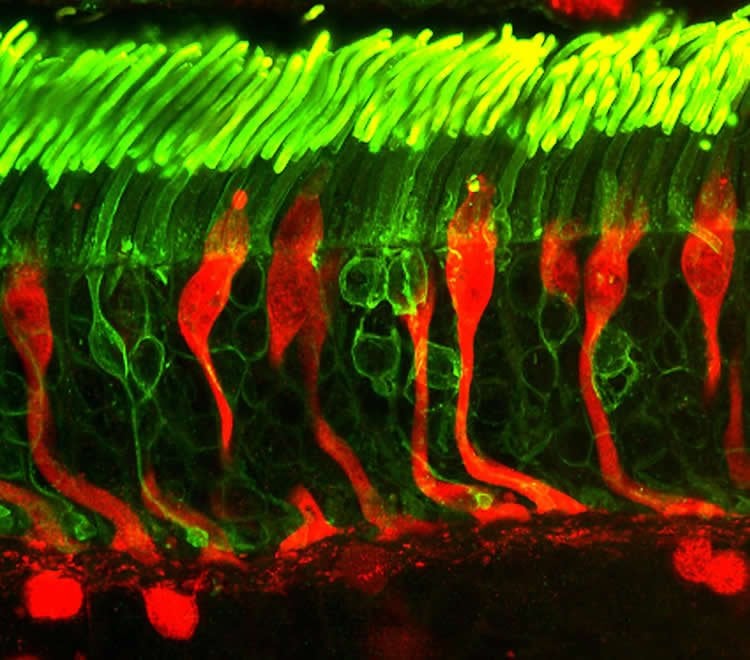
Next, the researchers looked across a broad sample of vertebrates (from the platypus to pouched and placental mammals) and compared their genomic sequences spanning the Nrl gene. They found that coding in the region of Nrl was highly conserved across the different species, indicating that the sequence for Nrl was well preserved through evolution – a sign that it was important for survival. Their data suggest that the first expression of Nrl appears to have originated among jawed vertebrates and that its expression occurred around the same time that rods became dominant in the retina.
Interestingly, similar patterns of cone activity were not found in lineage tracing of rods in the zebrafish retina. “That’s important,” said Swaroop “because zebrafish, with their cone-rich retinas, branched off earlier than mammals did from the evolutionary history of vertebrate.” The lack of cone lineage in zebrafish rods suggests that the origin of rod dominance, at the expense of cone cells, is a developmental innovation particular to mammals. Zebrafish rods may have emerged from a different mechanism, he said.
The findings may have relevance for current efforts to regenerate the retinal cells as a way of restoring vision in people with diseases, such as retinitis pigmentosa, that cause the retinal cells to degenerate. “Fish, which have cone-dominant retina are capable of regenerating their retina after injury. So if we want to understand how to regenerate retina in humans, we need to understand what happens in the fish. Understanding the evolution of photoreceptors is very much part of that,” he said.
Funding: The study was supported in part by the intramural research program of the NEI.
Source: Kathryn DeMott – NIH/National Eye Institute
Image Source: This NeuroscienceNews.com image is credited to National Eye Institute.
Original Research: Full open access research for “Recruitment of Rod Photoreceptors from Short-Wavelength-Sensitive Cones during the Evolution of Nocturnal Vision in Mammals” by Jung-Woong Kim, Hyun-Jin Yang, Adam Phillip Oel, Matthew John Brooks, Li Jia, David Charles Plachetzki, Wei Li, William Ted Allison, and Anand Swaroop in Developmental Cell. Published online June 20 2016 doi:10.1016/j.devcel.2016.05.023
[cbtabs][cbtab title=”MLA”]NIH/National Eye Institute. “Testing The Theory of How Rods in the Retina Originated.” NeuroscienceNews. NeuroscienceNews, 20 June 2016.
<https://neurosciencenews.com/retina-rods-neuroscience-4517/>.[/cbtab][cbtab title=”APA”]NIH/National Eye Institute. (2016, June 20). Testing The Theory of How Rods in the Retina Originated. NeuroscienceNews. Retrieved June 20, 2016 from https://neurosciencenews.com/retina-rods-neuroscience-4517/[/cbtab][cbtab title=”Chicago”]NIH/National Eye Institute. “Testing The Theory of How Rods in the Retina Originated.” https://neurosciencenews.com/retina-rods-neuroscience-4517/ (accessed June 20, 2016).[/cbtab][/cbtabs]
Abstract
Recruitment of Rod Photoreceptors from Short-Wavelength-Sensitive Cones during the Evolution of Nocturnal Vision in Mammals
Highlights
•Rod photoreceptors in mouse retina show genetic and epigenetic vestiges of S cones
•Rod photoreceptors in mice but not zebrafish exhibit S-cone lineage
•NRL locus of early mammals accrued regulatory elements driving rod-dominant retina
•Recruitment of S cones to rods in mammals helped mitigate the nocturnal bottleneck
Summary
Vertebrate ancestors had only cone-like photoreceptors. The duplex retina evolved in jawless vertebrates with the advent of highly photosensitive rod-like photoreceptors. Despite cones being the arbiters of high-resolution color vision, rods emerged as the dominant photoreceptor in mammals during a nocturnal phase early in their evolution. We investigated the evolutionary and developmental origins of rods in two divergent vertebrate retinas. In mice, we discovered genetic and epigenetic vestiges of short-wavelength cones in developing rods, and cell-lineage tracing validated the genesis of rods from S cones. Curiously, rods did not derive from S cones in zebrafish. Our study illuminates several questions regarding the evolution of duplex retina and supports the hypothesis that, in mammals, the S-cone lineage was recruited via the Maf-family transcription factor NRL to augment rod photoreceptors. We propose that this developmental mechanism allowed the adaptive exploitation of scotopic niches during the nocturnal bottleneck early in mammalian evolution.
“Recruitment of Rod Photoreceptors from Short-Wavelength-Sensitive Cones during the Evolution of Nocturnal Vision in Mammals” by Jung-Woong Kim, Hyun-Jin Yang, Adam Phillip Oel, Matthew John Brooks, Li Jia, David Charles Plachetzki, Wei Li, William Ted Allison, and Anand Swaroop in Developmental Cell. Published online June 20 2016 doi:10.1016/j.devcel.2016.05.023