Salk researchers discover a master gene responsible for sleep and wake cycles, offering hope for a drug that could help reset sleep.
Scientists at the Salk Institute for Biological Studies have identified a gene that regulates sleep and wake rhythms.
The discovery of the role of this gene, called Lhx1, provides scientists with a potential therapeutic target to help night-shift workers or jet lagged travelers adjust to time differences more quickly. The results, published in eLife, can point to treatment strategies for sleep problems caused by a variety of disorders.
“It’s possible that the severity of many dementias comes from sleep disturbances,” says Satchidananda Panda, a Salk associate professor who led the research team. “If we can restore normal sleep, we can address half of the problem.”
Every cell in the body has a “clock” – an abundance of proteins that dip or rise rhythmically over approximately 24 hours. The master clock responsible for establishing these cyclic circadian rhythms and keeping all the body’s cells in sync is the suprachiasmatic nucleus (SCN), a small, densely packed region of about 20,000 neurons housed in the brain’s hypothalamus.
More so than in other areas of the brain, the SCN’s neurons are in close and constant communication with one another. This close interaction, combined with exposure to light and darkness through vision circuits, keeps this master clock in sync and allows people to stay on essentially the same schedule every day. The tight coupling of these cells also helps make them collectively resistant to change. Exposure to light resets less than half of the SCN cells, resulting in long periods of jet lag.
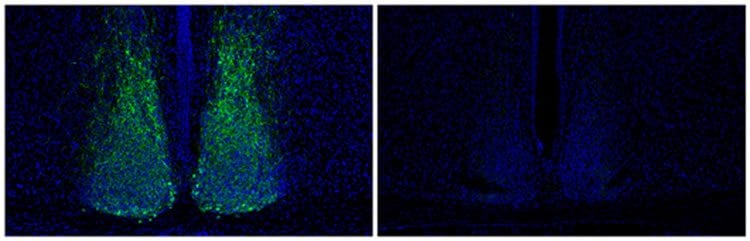
In the new study, researchers disrupted the light-dark cycles in mice and compared changes in the expression of thousands of genes in the SCN with other mouse tissues. They identified 213 gene expression changes that were unique to the SCN and narrowed in on 13 of these that coded for molecules that turn on and off other genes. Of those, only one was suppressed in response to light: Lhx1.
“No one had ever imagined that Lhx1 might be so intricately involved in SCN function,” says Shubhroz Gill, a postdoctoral researcher and co-first author of the paper. Lhx1 is known for its role in neural development: it’s so important, that mice without the gene do not survive. But this is the first time it has been identified as a master regulator of light-dark cycle genes.
By recording electrical activity in the SCN of animals with reduced amounts of the Lhx1 protein, the researchers saw that the SCN neurons weren’t in sync with one another, despite appearing rhythmic individually.
“It was all about communication–the neurons were not talking to each other without this molecule,” says Ludovic Mure, a postdoctoral researcher and an author on the paper. A next step in the work will be to understand exactly how Lhx1 affects the expression of genes that creates this synchronicity.
Studying a mouse version of jet lag–an 8-hour shift in their day-night cycle–the scientists found that those with little or no Lhx1 readjusted much faster to the shift than normal mice. This suggests that because these neurons are less in sync with one another, they are more easily able to shift to a new schedule, though it is difficult for them to maintain that schedule, Panda says.
These mice also exhibited reduced activity of certain genes, including one that creates vasoactive intestinal peptide or Vip, a molecule that has important roles in development and as a hormone in the intestine and blood. In the brain, Vip affects cell communication, but nobody had known that Lhx1 regulated it until now, Panda says. Interestingly, the team also found that adding Vip restored cell synchrony in the SCN.
“This approach helped us to close that knowledge gap and show that Vip is a very important protein, at least for SCN,” Panda says. “It can compensate for the loss of Lhx1.”
On the other hand, cutting back on Vip could be another way to treat jet lag. Vip could be an even easier drug target compared with Lhx1 because Vip is secreted from cells rather than inside cells, Panda says. “If we find a drug that will block the Vip receptor or somehow break down Vip, then maybe that will help us reset the clock much faster,” he adds.
The new results take the group a step closer to their goal of creating cell regenerative therapies that restore the SCN and ameliorate sleep problems. The scientists have made their gene expression data available through a searchable web interface, giving other researchers a handy way to explore the effect of light and dark in genes in the SCN and other tissues.
Other researchers on the study were Megumi Hatori, now at Keio University School of Medicine in Tokyo, and Martyn Goulding and Dennis D.M. O’Leary of Salk.
The work was supported by fellowships from The Japan Society for the Promotion of Science, Messinger Healthy Living, the Fyssen and Catharina Foundation, the Mary K. Chapman Foundation, The Leona M. and Harry B. Helmsley Charitable Trust, the National Institutes of Health, the Hearst Foundation and the Glenn Foundation.
Source Salk Communications – Salk Institute
Contact: Salk Institute press release
Image Source: The image is credited to Salk Institute and is adapted from the press release
Original Research Full open access research for “Lhx1 maintains synchrony among circadian oscillator neurons of the SCN” by Megumi Hatori, Shubhroz Gill, Ludovic S Mure, Martyn Goulding, Dennis DM O’Leary, Satchidananda Panda in eLife. Published online July 17 2014 doi:10.7554/eLife.03357
Lhx1 maintains synchrony among circadian oscillator neurons of the SCN
The robustness and limited plasticity of the master circadian clock in the suprachiasmatic nucleus (SCN) is attributed to strong intercellular communication among its constituent neurons. However, factors that specify this characteristic feature of the SCN are unknown. Here we identified Lhx1 as a regulator of SCN coupling. A phase-shifting light pulse causes acute reduction in Lhx1 expression and of its target genes that participate in SCN coupling. Mice lacking Lhx1 in the SCN have intact circadian oscillators, but reduced levels of coupling factors. Consequently, the mice rapidly phase shift under a jet lag paradigm and their behavior rhythms gradually deteriorate under constant condition. Ex vivo recordings of the SCN from these mice showed rapid desynchronization of unit oscillators. Therefore, by regulating expression of genes mediating intercellular communication, Lhx1 imparts synchrony among SCN neurons and ensures consolidated rhythms of activity and rest that is resistant to photic noise.
“Lhx1 maintains synchrony among circadian oscillator neurons of the SCN” by Megumi Hatori, Shubhroz Gill, Ludovic S Mure, Martyn Goulding, Dennis DM O’Leary, Satchidananda Panda in eLife, July 17 2014 doi:10.7554/eLife.03357.