Summary: A new study looks at how the digestive tract communicates with the brain and could help find new treatment options for obesity.
Source: Swiss National Science Foundation.
Our eating behaviour is influenced by the way our digestive tract communicates with our brain. If we can achieve a better understanding of the signalling pathways between them, it may help us find new treatments for obesity.
We eat because we’re hungry – and for a thousand other reasons too: for pleasure, out of frustration, or because we’re stressed. We’ve known for a long time that the digestive tract and the brain together determine our eating habits. And finding out how they do this is becoming increasingly relevant, given the current spread of overweight, obesity and type-2 diabetes.
However, the most efficient methods to reduce obesity today are all surgical interventions in the digestive tract: the gastric bypass, and the gastric sleeve that reduces stomach volume. “Surprisingly, while these alterations are completely different in anatomical terms, they have the same principal effect, namely a total and lasting rearrangement of the hormonal balance”, explains Ralph Peterli, a visceral surgeon and researcher at the Claraspital in Basel. His team first succeeded in proving this in 2009, in a sleeve gastrectomy case.
“And then there’s the role of the brain”, says Peterli. “It’s got to be involved, for instance, if patients suddenly don’t want fatty foods any more after their operation, but instead have an appetite for vegetables”. The researchers at the Claraspital are currently using functional magnetic resonance imaging (fMRI) to analyse how the brains of test subjects react to eating different foodstuffs.
Rats eat more, but less often
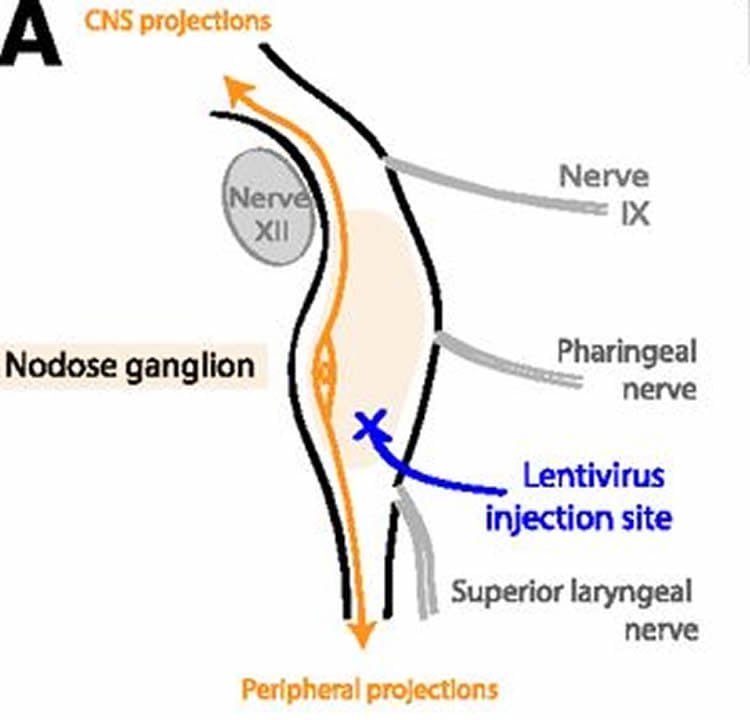
But how does the stomach actually send its signals to the brain? That’s what Wolfgang Langhans is trying to find out. He’s a physiologist at ETH Zurich. “If we knew this, it could help us to develop pharmacological strategies as an alternative to surgery – because surgery involves risks”, says Langhans. One of the topics of his research is the role of ‘glucagon-like peptide 1’ (GLP – 1), which has been known for some time to be an appetite-suppressing hormone. It is produced in large quantities as soon as the intestine is full of food. Just like all hormones, GLP-1 presumably travels through the bloodstream to the brain, where it has its impact. But Langhans and his team believe that GLP-1 also sends nerve signals by docking onto the GLP-1 receptors of the vagus nerve that connects the intestine to the brain.
In order to test their hypothesis, they used rats in which GLP-1 has the same function as in humans. The researchers injected viruses with genetically altered material – so-called viral vectors – into the vagus nerve of the animals. These viruses inhibit the production of GLP-1 receptors in the intestinal nerve cells. The number of receptors consequently fell by roughly half.
These downregulated GLP-1 nerve connections from the intestine to the brain indeed brought about a shift in eating behaviour. The rats ate for longer and ingested more at every mealtime, and afterwards they showed considerably higher levels of blood sugar. However, the amount that they ate each day did not increase. They ate more at any one time, but less often.
Surgery has a longer impact
“The result might seem perhaps a little disappointing”, says Langhans, “but in physiological terms it’s fascinating. It confirms the role of GLP-1 and the vagus nerve in achieving satiety, but it also shows that the control mechanism for the ingestion of food is highly robust”.
Peterli is also convinced of this. But this is precisely why he doubts that there will be a pharmacological alternative to his surgical procedures. “Surgery doesn’t just have an impact on one or two hormones. It influences fifty or a hundred mechanisms at the same time. We don’t even know most of them”. However, he too can imagine that hormone products or receptor blockers could support the impact of an operation. So the practical implementation of active substances seems imminent. But the day when science will be able to offer a comprehensive explanation of the multifarious connections between the intestine and the brain still seems far off.
Source: Stéphane Praz – Swiss National Science Foundation
Image Source: NeuroscienceNews.com image is credited to Krieger et al./Diabetes.
Original Research: Full open access research for “Knockdown of GLP-1 Receptors in Vagal Afferents Affects Normal Food Intake and Glycemia” by Jean-Philippe Krieger, Myrtha Arnold, Klaus G. Pettersen, Pius Lossel, Wolfgang Langhans and Shin J. Lee in Diabetes. Published online October 2016 doi:10.2337/db15-0973
[cbtabs][cbtab title=”MLA”]Swiss National Science Foundation “How the Stomach Talks to the Brain.” NeuroscienceNews. NeuroscienceNews, 10 October 2016.
<https://neurosciencenews.com/brain-stomach-obesity-5253/>.[/cbtab][cbtab title=”APA”]Swiss National Science Foundation (2016, October 10). How the Stomach Talks to the Brain. NeuroscienceNew. Retrieved October 10, 2016 from https://neurosciencenews.com/brain-stomach-obesity-5253/[/cbtab][cbtab title=”Chicago”]Swiss National Science Foundation “How the Stomach Talks to the Brain.” https://neurosciencenews.com/brain-stomach-obesity-5253/ (accessed October 10, 2016).[/cbtab][/cbtabs]
Abstract
Knockdown of GLP-1 Receptors in Vagal Afferents Affects Normal Food Intake and Glycemia
Nutrient stimulation of enteroendocrine L cells induces the release of the incretin and satiating peptide glucagon-like peptide 1 (GLP-1). The vagus nerve innervates visceral organs and may contribute to the mediation of gut-derived GLP-1’s effects on food intake, energy homeostasis, and glycemic control. To test the hypothesis that vagal afferent neuron (VAN) GLP-1 receptors (GLP-1Rs) are necessary for these effects of endogenous GLP-1, we established a novel bilateral nodose ganglia injection technique to deliver a lentiviral vector and to knock down VAN GLP-1Rs in male Sprague Dawley rats. We found that a full expression of VAN GLP-1Rs is not necessary for the maintenance of long-term energy balance in normal eating conditions. VAN GLP-1R knockdown (kd) did, however, increase meal size and accelerated gastric emptying. Moreover, postmeal glycemia was elevated and insulin release was blunted in GLP-1R kd rats, suggesting that VAN GLP-1Rs are physiological contributors to the neuroincretin effect after a meal. Collectively, our results highlight a crucial role for the VANs in mediating the effects of endogenous GLP-1 on food intake and glycemia and may promote the further development of GLP-1–based therapies.
“Knockdown of GLP-1 Receptors in Vagal Afferents Affects Normal Food Intake and Glycemia” by Jean-Philippe Krieger, Myrtha Arnold, Klaus G. Pettersen, Pius Lossel, Wolfgang Langhans and Shin J. Lee in Diabetes. Published online October 2016 doi:10.2337/db15-0973






