Summary: Researchers have successfully prevented early events that occur in the brain before Alzheimer’s symptoms are evident.
Source: Baylor College of Medicine.
Taking a pill that prevents the accumulation of toxic molecules in the brain might someday help prevent or delay Alzheimer’s disease, according to scientists at Baylor College of Medicine, Texas Children’s Hospital and Johns Hopkins University School of Medicine.
The study, published today in Cell Press journal Neuron, took a three-pronged approach to help subdue early events that occur in the brain long before symptoms of Alzheimer’s disease are evident. The scientists were able to prevent those early events and the subsequent development of brain pathology in experimental animal models in the lab.
“Common diseases like Parkinson’s, Alzheimer’s and dementia are caused in part by abnormal accumulation of certain proteins in the brain,” said senior author Dr. Huda Zoghbi, professor of molecular and human genetics and of pediatrics – neurology and developmental neuroscience at Baylor and director of the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital. “Some proteins become toxic when they accumulate; they make the brain vulnerable to degeneration. Tau is one of those proteins involved in Alzheimer’s disease and dementia.”
“Scientists in the field have been focusing mostly on the final stages of Alzheimer’s disease,” said first author Dr. Cristian Lasagna-Reeves, postdoctoral fellow in the Zoghbi lab. “Here we tried to find clues about what is happening at the very early stages of the illness, before clinical irreversible symptoms appear, with the intention of preventing or reducing those early events that lead to devastating changes in the brain decades later.”
The scientists reasoned that if they could find ways to prevent or reduce tau accumulation in the brain, they would uncover new possibilities for developing drug treatments for these diseases.
Cells control the amount of their proteins with other proteins called enzymes. To find which enzymes affect tau accumulation, the scientists systematically inhibited enzymes called kinases.
“We inhibited about 600 kinases one by one and found one, called Nuak1, whose inhibition resulted in reduced levels of tau,” said Zoghbi, who is also an investigator at the Howard Hughes Medical Institute.
The scientists screened the enzymes in two different systems, cultured human cells and the laboratory fruit fly. Screening in the fruit fly allowed the scientists to assess the effects of inhibiting the enzymes in a functional nervous system in a living organism.
“Screening hundreds of kinases in the fruit fly animal model was critical because we could assess degeneration caused by tau in the fly’s nervous system and measure neuronal dysfunction. Screening such a large number cannot be done with other animal models like the mouse, and cultured cells cannot model complex nervous system functions,” said co-senior author Dr. Juan Botas, professor of molecular and human genetics and of molecular and cellular biology at Baylor.
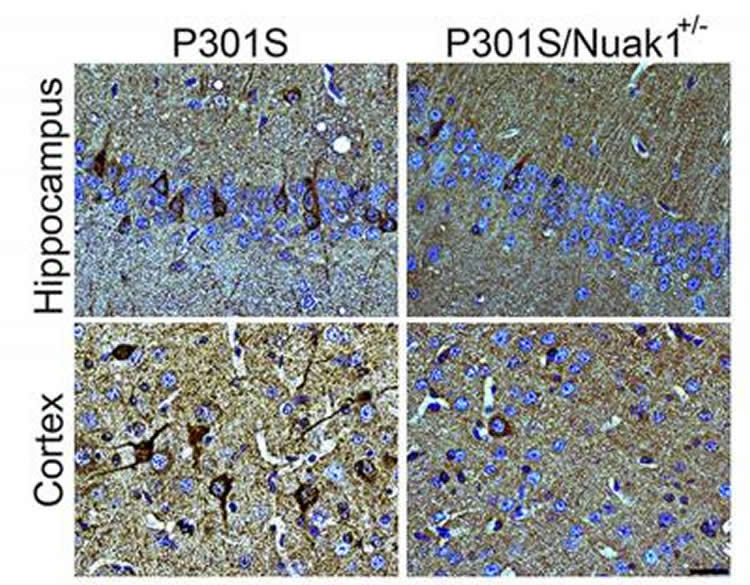
Brain section from mouse carrying the dementia-causing P301S mutation in human tau shows accumulation of tau neurofibrillary tangles (in dark brown, left). When Nuak1 levels are decreased by 50% (P301S/Nuak1+/-; right), fewer tau tangles accumulate.
“We found one enzyme, Nuak1, whose inhibition consistently resulted in lower levels of tau in both human cells and fruit flies,” said Zoghbi. “Then we took this result to a mouse model of Alzheimer’s disease and hoped that the results would hold, and they did. Inhibiting Nuak1 improved the behavior of the mice and prevented brain degeneration.”
“Confirming in three independent systems – human cells, the fruit fly and the mouse – that Nuak1 inhibition results in reduced levels of tau and prevents brain abnormalities induced by tau accumulation, has convinced us that Nuak1 is a reliable potential target for drugs to prevent diseases such as Alzheimer’s,” said Zoghbi. “The next step is to develop drugs that will inhibit Nuak1 in hope that one day would be able to lower tau levels with low toxicity in individuals at risk for dementia due to tau accumulation.”
Scientific studies like this one that uncover basic biological mechanisms of disease make it possible to develop new strategies to prevent or treat diseases such as Alzheimer’s, Parkinson’s or dementia.
In the future it might be possible to treat people at risk for Alzheimer’s disease by keeping tau low. Think of how taking drugs that lower cholesterol has helped control the accumulation of cholesterol in blood vessels that leads to atherosclerosis and heart disease.
“When people started taking drugs that lower cholesterol, they lived longer and healthier lives rather than dying earlier of heart disease,” said Zoghbi. “Nobody has thought about Alzheimer’s disease in that light. Tau in Alzheimer’s can be compared to cholesterol in heart disease. Tau is a protein that when it accumulates as the person ages, increases the vulnerability of the brain to developing Alzheimer’s. So maybe if we can find drugs that can keep tau at levels that are not toxic for the brain, then we would be able to prevent or delay the development of Alzheimer’s and other diseases caused in part by toxic tau accumulation.”
“Just like people now take their cholesterol-lowering medications, people in the future could be taking medications to keep tau levels low and prevent the development of Alzheimer’s disease,” said Lasagna-Reeves.
Other contributors to this work include María de Haro, Shuang Hao, Jeehye Park, Maxime W.C. Rousseaux, Ismael Al-Ramahi, Paymaan Jafar-Nejad, Luis Vilanova-Velez, Lauren See, Antonia De Maio, Larissa Nitschke, Zhenyu Wu, Juan C. Troncoso, Thomas F. Westbrook and Jianrong Tang.
Funding: This work was supported by the Howard Hughes Medical Institute, the Robert A. and Renee E. Belfer Family Foundation, the Hamill Foundation, the Chapman Foundation, and the National Institutes of Health grants NIH/NINDS R01 NS027699-17, NIH/NINDS 3R01 NS027699-25S1 and 1K22NS092688-0. Support also was provided by the Texas Alzheimer’s Research and Care Consortium-Investigator Grant Program, the Darrel K. Royal Foundation grant, the Canadian Institutes of Health Research Fellowship (201210MFE-290072-173743), the Mass Spectrometry-Proteomics Core Laboratory (MS-PCL) and the confocal microscopy, neuroconnectivity and mouse behavioral cores of the Baylor College of Medicine Intellectual and Developmental Disabilities Research Center (1U54 HD083092), the Johns Hopkins University Morris Udall Parkinson’s Disease Center of Excellence (NINDS P50 NS38377) and Alzheimer Disease Research Center (NIA P50 AG05146).
Source: Graciela Gutierrez – Baylor College of Medicine
Image Source: NeuroscienceNews.com image is credited to the researchers.
Original Research: Abstract for “Reduction of Nuak1 Decreases Tau and Reverses Phenotypes in a Tauopathy Mouse Model” by Cristian A. Lasagna-Reeves, Maria de Haro, Shuang Hao, Jeehye Park, Maxime W.C. Rousseaux, Ismael Al-Ramahi, Paymaan Jafar-Nejad, Luis Vilanova-Velez, Lauren See, Antonia De Maio, Larissa Nitschke, Zhenyu Wu, Juan C. Troncoso, Thomas F. Westbrook, Jianrong Tang, Juan Botas, and Huda Y. Zoghbi in Neuron. Published online October 6 2016 doi:10.1016/j.neuron.2016.09.022
[cbtabs][cbtab title=”MLA”]Baylor College of Medicine. “A Potential New Strategy to Prevent Alzheimer’s.” NeuroscienceNews. NeuroscienceNews, 22 October 2016.
<https://neurosciencenews.com/alzheimers-nuak1-neurology-5328/>.[/cbtab][cbtab title=”APA”]Baylor College of Medicine. (2016, October 22). A Potential New Strategy to Prevent Alzheimer’s. NeuroscienceNews. Retrieved October 22, 2016 from https://neurosciencenews.com/alzheimers-nuak1-neurology-5328/[/cbtab][cbtab title=”Chicago”]Baylor College of Medicine. “A Potential New Strategy to Prevent Alzheimer’s.” https://neurosciencenews.com/alzheimers-nuak1-neurology-5328/ (accessed October 22, 2016).[/cbtab][/cbtabs]
Abstract
Reduction of Nuak1 Decreases Tau and Reverses Phenotypes in a Tauopathy Mouse Model
Highlights
•The AMPK-related kinase Nuak1 regulates tau levels
•Nuak1 is associated with tau pathology in AD and PSP patients
•Nuak1 directly phosphorylates tau at serine 356
•Reduction of Nuak1 rescues the phenotypes in tauopathy models
Summary
Many neurodegenerative proteinopathies share a common pathogenic mechanism: the abnormal accumulation of disease-related proteins. As growing evidence indicates that reducing the steady-state levels of disease-causing proteins mitigates neurodegeneration in animal models, we developed a strategy to screen for genes that decrease the levels of tau, whose accumulation contributes to the pathology of both Alzheimer disease (AD) and progressive supranuclear palsy (PSP). Integrating parallel cell-based and Drosophila genetic screens, we discovered that tau levels are regulated by Nuak1, an AMPK-related kinase. Nuak1 stabilizes tau by phosphorylation specifically at Ser356. Inhibition of Nuak1 in fruit flies suppressed neurodegeneration in tau-expressing Drosophila, and Nuak1 haploinsufficiency rescued the phenotypes of a tauopathy mouse model. These results demonstrate that decreasing total tau levels is a valid strategy for mitigating tau-related neurodegeneration and reveal Nuak1 to be a novel therapeutic entry point for tauopathies.
“Reduction of Nuak1 Decreases Tau and Reverses Phenotypes in a Tauopathy Mouse Model” by Cristian A. Lasagna-Reeves, Maria de Haro, Shuang Hao, Jeehye Park, Maxime W.C. Rousseaux, Ismael Al-Ramahi, Paymaan Jafar-Nejad, Luis Vilanova-Velez, Lauren See, Antonia De Maio, Larissa Nitschke, Zhenyu Wu, Juan C. Troncoso, Thomas F. Westbrook, Jianrong Tang, Juan Botas, and Huda Y. Zoghbi in Neuron. Published online October 6 2016 doi:10.1016/j.neuron.2016.09.022






