Summary: A study using male mice with a specific genetic mutation provides an innovative way to study how genes affect vocal sound sequencing.
Source: Duke University.
Woo-pitching song ends up scrambled because of mutation
There’s a particular order to the sounds of the ultrasonic song a male mouse performs to impress his potential mate.
But for male mice carrying a genetic mutation known to affect human speech, it is difficult to get this syntax of sounds right, according to a new study. Humans with the same mutation have problems with correctly sequencing phonemes into words.
“Mouse songs are not an exact parallel to human speech, but we found something very robust,” said Erich Jarvis, a professor of neurobiology at Duke University and Howard Hughes Medical Institute investigator who co-led the study appearing online in Frontiers in Behavioral Neuroscience.
Jarvis collaborated with Duke postdoctoral researcher Jonathan Chabout and Simon Fisher, director of the Max Planck Institute for Psycholinguistics in Nijmegen, the Netherlands.
“This work provides an innovative way to study how genes affect sequencing of vocal sounds,” said Fisher, who is also a professor of language and genetics at Nijmegen’s Donders Institute. “It’s important because quite a few children have mysterious problems with learning to produce proficient speech, and we have found that genetic factors play a big role. The big challenge, then, is to understand exactly why damage to a particular gene can lead to those kinds of difficulties, and that is hard to do if we can only investigate humans.”
The Duke researchers recorded ultrasonic sounds (above the range of human hearing) made by 50 adult male mice under a variety of conditions. They deciphered the structure of these mouse songs using new statistical tools to identify the ways four basic ‘syllables’ were strung together into more complicated sequences. They analyzed how these sequences (the syntax of the songs) changed in different social situations, such as when the male was in the presence of a female instead of another male.
Their goal was to see whether mutation of the gene Foxp2 (forkhead-box P2) can affect the sequencing of the mouse songs, as it is known to do in human speech.
Foxp2 has been the subject of intensive research in the 15 years since it was identified by Fisher and others as a key to mastering the rapid coordinated sequences of mouth, face and larynx (voice-box) movements that enable fluent human speech.
“We first found a mutation in this gene causing speech deficits in many relatives of a large British family,” Fisher said. “They made errors when speaking and these became worse as the things they were trying to say got longer and more complicated.”
Scientists using mice to understand how Foxp2 can affect vocal behaviors have mainly focused on the acoustic structure of individual syllables in juvenile mice, which have more basic vocalizations. The new study by Jarvis and his team instead looked carefully into the sequence of syllables in the songs of mature adults.
Super high-pitched mouse singing was identified decades ago, but only recently has it been possible to assign patterns to the chirping noises. In a 2012 paper appearing in PLoS One, the Duke team first proposed that mice have a limited version of the vocal learning brain structures and some of the vocal adaptability found in song-learning birds and humans.
Follow-up work appearing last year in Frontiers in Behavioral Neuroscience showed that male mice change their tune in response to different social situations — especially the presence of active females.
In this most recent study, the Duke team found that the songs of the mice become significantly more complicated in the presence of an active female than they do for a sleeping female, female urine or a sleeping male.
“Social context matters,” Jarvis said. “You have to be careful about the social context in which you record the mouse’s behavior.”
Half of the male mice in the current study were genetically engineered to carry a mutation identical to the one that Fisher and colleagues had discovered causing speech problems in humans. Those mice did not switch to the more complex syntax in the presence of the live female, almost as if they were “tongue-tied,” Jarvis said. These findings are consistent with other newly emerging studies of mice with different mutations of the gene.
“So, while the mice aren’t an exact model, and unlike humans they don’t seem to learn their vocalizations, we did find that this mutation is having a similar impact on the sequence of ultrasonic songs,” Jarvis said.
But the researchers didn’t have a way to statistically quantify changes in syntax, even for human speech. Jarvis and Chabout worked with Duke statisticians David Dunston and Abhra Sarkar to develop new tools to analyze the mouse syntax.
“If we’re looking for the effect of a gene, we need real statistical power,” Jarvis said. “It’s not enough to just eyeball it.”
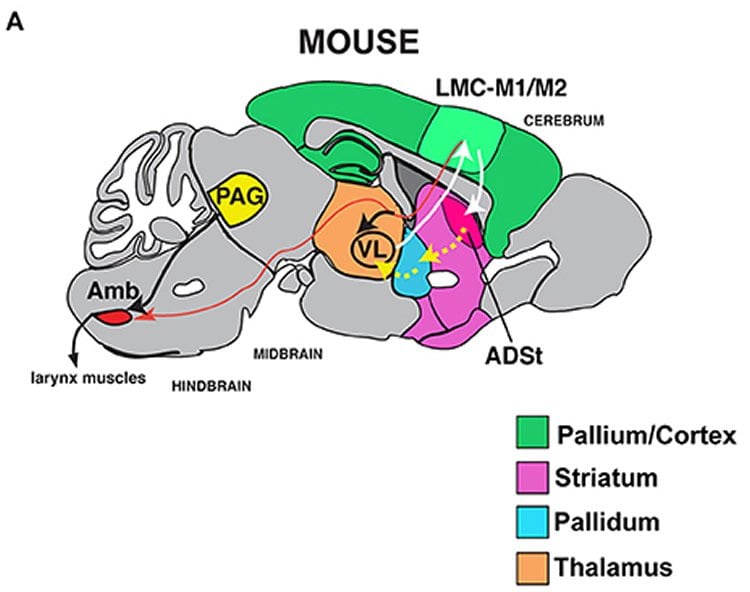
The team also traced the location of neurons that connect the muscles of the voice box with the higher parts of the brain involved in controlling movements. In mice with the Foxp2 mutation, the neurons were spread out in a wider pattern in this part of the brain.
Because Foxp2 is a transcription factor — a gene that tells other genes what to do — this altered pattern of neurons suggests it may play a role in how neurons are routed across the brain, Jarvis said. But he emphasized that more research is needed on that question.
The finding expands the usefulness of mice for studying human speech and the brain, Jarvis said. Though he and others have made tremendous progress by studying the brains of songbirds that can learn songs the way we learn words, “having a mammalian model with even some rudimentary genetics and connectivity for vocal communication could still get you closer to the simpler aspects of speech,” Jarvis said.
Funding: This research was supported by the Howard Hughes Medical Institute, the Max Planck Society and the U.S. Office of Naval Research (N00014-14-1-0245).
Source: Karl Leif Bates – Duke University
Image Source: NeuroscienceNews.com image is credited to the researchers/Frontiers in Behavioral Neuroscience.
Original Research: Full open access research for “A Foxp2 Mutation Implicated in Human Speech Deficits Alters Sequencing of Ultrasonic Vocalizations in Adult Male Mice” by Jonathan Chabout, Abhra Sarkar, Sheel R. Patel1, Taylor Radden, David B. Dunson, Simon E. Fisher and Erich D. Jarvis in Frontiers in Behavioral Neuroscience. Published online October 20 2016 doi:10.3389/fnbeh.2016.00197
[cbtabs][cbtab title=”MLA”]Duke University. “Male Mice Model Human Speech Defect.” NeuroscienceNews. NeuroscienceNews, 20 October 2016.
<https://neurosciencenews.com/woo-song-mouse-speech-5313/>.[/cbtab][cbtab title=”APA”]Duke University. (2016, October 20). Male Mice Model Human Speech Defect. NeuroscienceNews. Retrieved October 20, 2016 from https://neurosciencenews.com/woo-song-mouse-speech-5313/[/cbtab][cbtab title=”Chicago”]Duke University. “Male Mice Model Human Speech Defect.” https://neurosciencenews.com/woo-song-mouse-speech-5313/ (accessed October 20, 2016).[/cbtab][/cbtabs]
Abstract
A Foxp2 Mutation Implicated in Human Speech Deficits Alters Sequencing of Ultrasonic Vocalizations in Adult Male Mice
Development of proficient spoken language skills is disrupted by mutations of the FOXP2 transcription factor. A heterozygous missense mutation in the KE family causes speech apraxia, involving difficulty producing words with complex learned sequences of syllables. Manipulations in songbirds have helped to elucidate the role of this gene in vocal learning, but findings in non-human mammals have been limited or inconclusive. Here, we performed a systematic study of ultrasonic vocalizations (USVs) of adult male mice carrying the KE family mutation. Using novel statistical tools, we found that Foxp2 heterozygous mice did not have detectable changes in USV syllable acoustic structure, but produced shorter sequences and did not shift to more complex syntax in social contexts where wildtype animals did. Heterozygous mice also displayed a shift in the position of their rudimentary laryngeal motor cortex (LMC) layer-5 neurons. Our findings indicate that although mouse USVs are mostly innate, the underlying contributions of FoxP2 to sequencing of vocalizations are conserved with humans.
“A Foxp2 Mutation Implicated in Human Speech Deficits Alters Sequencing of Ultrasonic Vocalizations in Adult Male Mice” by Jonathan Chabout, Abhra Sarkar, Sheel R. Patel1, Taylor Radden, David B. Dunson, Simon E. Fisher and Erich D. Jarvis in Frontiers in Behavioral Neuroscience. Published online October 20 2016 doi:10.3389/fnbeh.2016.00197